Immunosuppression in early postnatal days induces persistent and allergen-specific immune tolerance to asthma in adult mice
- PMID: 25860995
- PMCID: PMC4393286
- DOI: 10.1371/journal.pone.0122990
Immunosuppression in early postnatal days induces persistent and allergen-specific immune tolerance to asthma in adult mice
Abstract
Bronchial asthma is a chronic airway inflammatory condition with high morbidity, and effective treatments for asthma are limited. Allergen-specific immunotherapy can only induce peripheral immune tolerance and is not sustainable. Exploring new therapeutic strategies is of great clinical importance. Recombinant adenovirus (rAdV) was used as a vector to make cells expressing cytotoxic T lymphocyte-associated antigen-4-immunoglobulin (CTLA4Ig) a soluble CTLA4 immunoglobulin fusion protein. Dendritic cells (DCs) were modified using the rAdVs together with allergens. Then these modified DCs were transplanted to mice before allergen sensitization. The persistence and specificity of immune tolerance were evaluated in mice challenged with asthma allergens at 3 and 7 months. DCs modified by CTLA4Ig showed increased IL-10 secretion, decreased IL-12 secretion, and T cell stimulation in vitro. Mice treated with these DCs in the early neonatal period developed tolerance against the allergens that were used to induce asthma in the adult stage. Asthma symptoms, lung damage, airway reactivity, and inflammatory response all improved. Humoral immunity indices showed that this therapeutic strategy strongly suppressed mice immune responses and was maintained for as long as 7 months. Furthermore, allergen cross-sensitization and challenge experiments demonstrated that this immune tolerance was allergen-specific. Treatment with CTLA4Ig modified DCs in the early neonatal period, inducing persistent and allergen-specific immune tolerance to asthma in adult mice. Our results suggest that it may be possible to develop a vaccine for asthma.
Conflict of interest statement
Figures
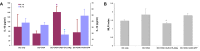
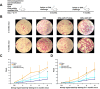
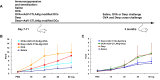
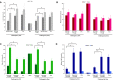
Similar articles
-
T lymphocyte antigen 4-modified dendritic cell therapy for asthmatic mice guided by the CCR7 chemokine receptor.Int J Mol Sci. 2014 Aug 29;15(9):15304-19. doi: 10.3390/ijms150915304. Int J Mol Sci. 2014. PMID: 25177863 Free PMC article.
-
Induction of immune tolerance in asthmatic mice by vaccination with DNA encoding an allergen-cytotoxic T lymphocyte-associated antigen 4 combination.Clin Vaccine Immunol. 2011 May;18(5):807-14. doi: 10.1128/CVI.00434-10. Epub 2011 Feb 23. Clin Vaccine Immunol. 2011. PMID: 21346053 Free PMC article.
-
Cytokine gene-modulated dendritic cells protect against allergic airway inflammation by inducing IL-10(+)IFN-gamma(+)CD4(+) T cells.Gene Ther. 2010 Aug;17(8):1011-21. doi: 10.1038/gt.2010.39. Epub 2010 Apr 1. Gene Ther. 2010. PMID: 20357831
-
Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens.Ann Allergy Asthma Immunol. 2018 Sep;121(3):306-312. doi: 10.1016/j.anai.2018.06.026. Epub 2018 Jun 30. Ann Allergy Asthma Immunol. 2018. PMID: 29966703 Review.
-
T-cell tolerance to inhaled allergens: mechanisms and therapeutic approaches.Expert Opin Biol Ther. 2008 Jun;8(6):769-77. doi: 10.1517/14712598.8.6.769. Expert Opin Biol Ther. 2008. PMID: 18476788 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

