The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach
- PMID: 25861386
- PMCID: PMC4380922
- DOI: 10.1111/eva.12202
The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach
Abstract
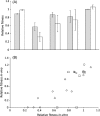
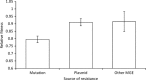
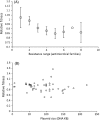
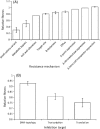
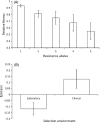
The evolution of antibiotic resistance carries a fitness cost, expressed in terms of reduced competitive ability in the absence of antibiotics. This cost plays a key role in the dynamics of resistance by generating selection against resistance when bacteria encounter an antibiotic-free environment. Previous work has shown that the cost of resistance is highly variable, but the underlying causes remain poorly understood. Here, we use a meta-analysis of the published resistance literature to determine how the genetic basis of resistance influences its cost. We find that on average chromosomal resistance mutations carry a larger cost than acquiring resistance via a plasmid. This may explain why resistance often evolves by plasmid acquisition. Second, we find that the cost of plasmid acquisition increases with the breadth of its resistance range. This suggests a potentially important limit on the evolution of extensive multidrug resistance via plasmids. We also find that epistasis can significantly alter the cost of mutational resistance. Overall, our study shows that the cost of antimicrobial resistance can be partially explained by its genetic basis. It also highlights both the danger associated with plasmidborne resistance and the need to understand why resistance plasmids carry a relatively low cost.
Keywords: adaptation; antibiotic resistance; antimicrobial resistance; fitness cost; microbes; mutation; plasmid.
Figures



References
-
- Abdelraouf K, Kabbara S, Ledesma KR, Poole K. Tam VH. Effect of multidrug resistance-conferring mutations on the fitness and virulence of Pseudomonas aeruginosa. Journal of Antimicrobial Chemotherapy. 2011;66:1311–1317. - PubMed
-
- Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, Vankerckhoven V, et al. European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997–2009) Journal of Antimicrobial Chemotherapy. 2011;66:vi3–vi12. - PubMed
-
- Alekshun MN. Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. - PubMed
-
- Andersson DI. The biological cost of mutational antibiotic resistance: any practical conclusions? Current Opinion in Microbiology. 2006;9:461–465. - PubMed
-
- Andersson DI. Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nature Reviews Microbiology. 2010;8:260–271. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

