From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition?
- PMID: 25861393
- PMCID: PMC4389409
- DOI: 10.1186/s13148-015-0068-2
From inflammaging to healthy aging by dietary lifestyle choices: is epigenetics the key to personalized nutrition?
Abstract
The progressively older population in developed countries is reflected in an increase in the number of people suffering from age-related chronic inflammatory diseases such as metabolic syndrome, diabetes, heart and lung diseases, cancer, osteoporosis, arthritis, and dementia. The heterogeneity in biological aging, chronological age, and aging-associated disorders in humans have been ascribed to different genetic and environmental factors (i.e., diet, pollution, stress) that are closely linked to socioeconomic factors. The common denominator of these factors is the inflammatory response. Chronic low-grade systemic inflammation during physiological aging and immunosenescence are intertwined in the pathogenesis of premature aging also defined as 'inflammaging.' The latter has been associated with frailty, morbidity, and mortality in elderly subjects. However, it is unknown to what extent inflammaging or longevity is controlled by epigenetic events in early life. Today, human diet is believed to have a major influence on both the development and prevention of age-related diseases. Most plant-derived dietary phytochemicals and macro- and micronutrients modulate oxidative stress and inflammatory signaling and regulate metabolic pathways and bioenergetics that can be translated into stable epigenetic patterns of gene expression. Therefore, diet interventions designed for healthy aging have become a hot topic in nutritional epigenomic research. Increasing evidence has revealed that complex interactions between food components and histone modifications, DNA methylation, non-coding RNA expression, and chromatin remodeling factors influence the inflammaging phenotype and as such may protect or predispose an individual to many age-related diseases. Remarkably, humans present a broad range of responses to similar dietary challenges due to both genetic and epigenetic modulations of the expression of target proteins and key genes involved in the metabolism and distribution of the dietary constituents. Here, we will summarize the epigenetic actions of dietary components, including phytochemicals, and macro- and micronutrients as well as metabolites, that can attenuate inflammaging. We will discuss the challenges facing personalized nutrition to translate highly variable interindividual epigenetic diet responses to potential individual health benefits/risks related to aging disease.
Keywords: Aging; Epigenetics; Inflammation; Metabolism; Oxidative stress; Personalized nutrition; Phytochemicals.
Figures
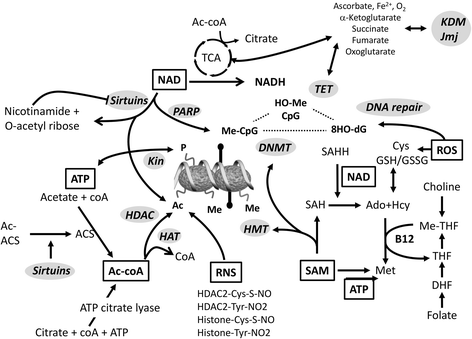
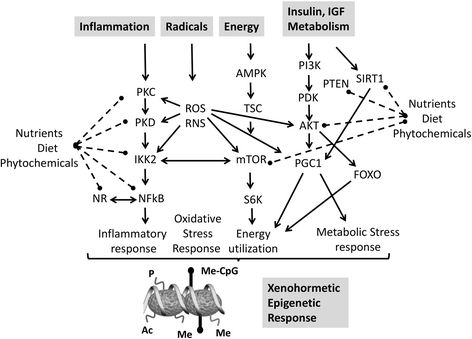
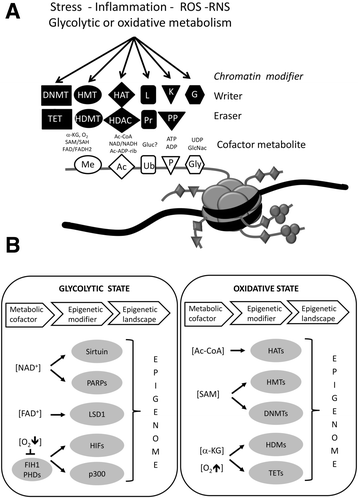
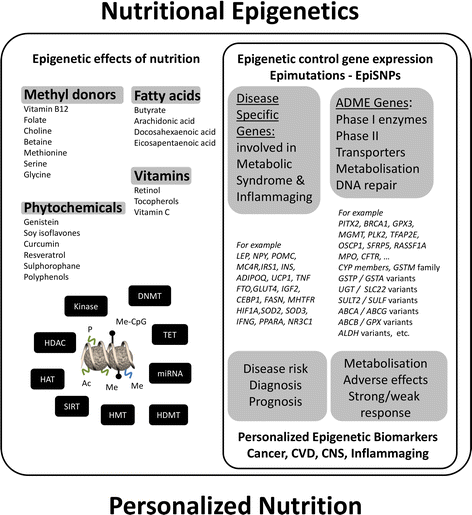
References
-
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. - PubMed
-
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflammaging. Curr Pharm Des. 2009;15(26):3003–26. - PubMed
-
- Ling C, Ronn T. Epigenetic adaptation to regular exercise in humans. Drug Discov Today. 2014;19(7):1015–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

