Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus
- PMID: 25861456
- PMCID: PMC4379887
- DOI: 10.1136/lupus-2014-000074
Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus
Abstract
Objective: We investigated whether systemic lupus erythematosus (SLE) disease duration or serology associate with abnormal regional glucose metabolism as measured with [(18)F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) and deficits on neuropsychological testing.
Methods: Subjects with SLE with stable disease activity, without brain damage or clinical symptoms of neuropsychiatric (NP) SLE, stratified by disease duration (short-term (ST)-SLE=disease ≤2 years, long-term (LT)-SLE=disease ≥10 years), underwent clinical assessments, neuropsychological testing, resting FDG-PET scan imaging and measurement of serum titres of antibody to N-methyl-d-aspartate receptor (DNRAb). FDG-PET scans were compared with age-matched and gender-matched healthy controls.
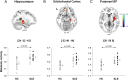
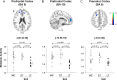
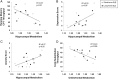
Results: Subjects with LT-SLE demonstrated hypometabolism in the prefrontal and premotor cortices that correlated with accrued SLE-related damage, but not with DNRAb titre or performance on NP testing. Independent of disease duration, subjects with SLE demonstrated hypermetabolism in the hippocampus and orbitofrontal cortex that correlated with impaired memory performance and mood alterations (depression, anxiety, fatigue). Serum DNRAb also correlated independently with impaired memory performance and increased anxiety. Together, serum DNRAb titre and regional hypermetabolism were more powerful predictors of performance than either alone.
Interpretation: The presence of serum DNRAbs can account for some aspects of brain dysfunction in patients with SLE, and the addition of regional measurements of resting brain metabolism improves the assessment and precise attribution of central nervous system manifestations related to SLE.
Keywords: Autoantibodies; Autoimmune Diseases; Systemic Lupus Erythematosus.
Figures

References
-
- Ainiala H, Loukkola J, Peltola J et al. . The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001;57:496–500 doi:10.1212/WNL.57.3.496 - DOI - PubMed
-
- Brey RL, Holliday SL, Saklad AR et al. . Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002;58:1214–20 doi:10.1212/WNL.58.8.1214 - DOI - PubMed
-
- Nowicka-Sauer K, Czuszynska Z, Smolenska Z et al. . Neuropsychological assessment in systemic lupus erythematosus patients: clinical usefulness of first-choice diagnostic tests in detecting cognitive impairment and preliminary diagnosis of neuropsychiatric lupus. Clin Exp Rheumatol 2011;29:299–306. - PubMed
-
- Unterman A, Nolte JES, Boaz M et al. . Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 2011;41:1–11 doi:10.1016/j.semarthrit.2010.08.001 - DOI - PubMed
-
- Stojanovich L, Zandman-Goddard G, Pavlovich S et al. . Psychiatric manifestations in systemic lupus erythematosus. Autoimmun Rev 2007;6:421–6 doi:10.1016/j.autrev.2007.02.007 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
