Structure-activity relationship studies of the lipophilic tail region of sphingosine kinase 2 inhibitors
- PMID: 25862200
- PMCID: PMC4580500
- DOI: 10.1016/j.bmcl.2015.03.041
Structure-activity relationship studies of the lipophilic tail region of sphingosine kinase 2 inhibitors
Abstract
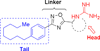
Sphingosine-1-phosphate (S1P) is a ubiquitous, endogenous small molecule that is synthesized by two isoforms of sphingosine kinase (SphK1 and 2). Intervention of the S1P signaling pathway has attracted significant attention because alteration of S1P levels is linked to several disease states including cancer, fibrosis, and sickle cell disease. While intense investigations have focused on developing SphK1 inhibitors, only a limited number of SphK2-selective agents have been reported. Herein, we report our investigations on the structure-activity relationship studies of the lipophilic tail region of SLR080811, a SphK2-selective inhibitor. Our studies demonstrate that the internal phenyl ring is a key structural feature that is essential in the SLR080811 scaffold. Further, we show the dependence of SphK2 activity and selectivity on alkyl tail length, suggesting a larger lipid binding pocket in SphK2 compared to SphK1.
Keywords: S1P; SphK1; SphK2; Sphingosine kinase; Sphingosine-1-phosphate.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

