New insights into the mechanism of DNA mismatch repair
- PMID: 25862369
- PMCID: PMC4600670
- DOI: 10.1007/s00412-015-0514-0
New insights into the mechanism of DNA mismatch repair
Abstract
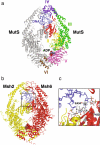
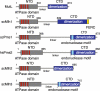
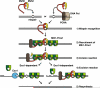
The genome of all organisms is constantly being challenged by endogenous and exogenous sources of DNA damage. Errors like base:base mismatches or small insertions and deletions, primarily introduced by DNA polymerases during DNA replication are repaired by an evolutionary conserved DNA mismatch repair (MMR) system. The MMR system, together with the DNA replication machinery, promote repair by an excision and resynthesis mechanism during or after DNA replication, increasing replication fidelity by up-to-three orders of magnitude. Consequently, inactivation of MMR genes results in elevated mutation rates that can lead to increased cancer susceptibility in humans. In this review, we summarize our current understanding of MMR with a focus on the different MMR protein complexes, their function and structure. We also discuss how recent findings have provided new insights in the spatio-temporal regulation and mechanism of MMR.
Figures


References
-
- Acharya S, Foster PL, Brooks P, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol Cell. 2003;12:233–246. - PubMed
-
- Ahrends R, Kosinski J, Kirsch D, Manelyte L, Giron-Monzon L, Hummerich L, Schulz O, Spengler B, Friedhoff P. Identifying an interaction site between MutH and the C-terminal domain of MutL by crosslinking, affinity purification, chemical coding and mass spectrometry. Nucleic Acids Res. 2006;34:3169–3180. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

