MBL-associated serine proteases (MASPs) and infectious diseases
- PMID: 25862418
- PMCID: PMC7112674
- DOI: 10.1016/j.molimm.2015.03.245
MBL-associated serine proteases (MASPs) and infectious diseases
Abstract
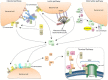
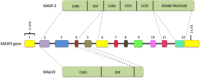
The lectin pathway of the complement system has a pivotal role in the defense against infectious organisms. After binding of mannan-binding lectin (MBL), ficolins or collectin 11 to carbohydrates or acetylated residues on pathogen surfaces, dimers of MBL-associated serine proteases 1 and 2 (MASP-1 and MASP-2) activate a proteolytic cascade, which culminates in the formation of the membrane attack complex and pathogen lysis. Alternative splicing of the pre-mRNA encoding MASP-1 results in two other products, MASP-3 and MAp44, which regulate activation of the cascade. A similar mechanism allows the gene encoding MASP-2 to produce the truncated MAp19 protein. Polymorphisms in MASP1 and MASP2 genes are associated with protein serum levels and functional activity. Since the first report of a MASP deficiency in 2003, deficiencies in lectin pathway proteins have been associated with recurrent infections and several polymorphisms were associated with the susceptibility or protection to infectious diseases. In this review, we summarize the findings on the role of MASP polymorphisms and serum levels in bacterial, viral and protozoan infectious diseases.
Keywords: Complement system; MASP-1; MASP-2; MASP-3; MAp19; MAp44.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abbas A.K., Lichtman A.H., Pillai S. 7 ed. EUA: Sauders Elsevier Inc.; 2012. Cellular and Molecular Immunology.
-
- Ali Y.M., Lynch N.J., Haleem K.S., Fujita T., Endo Y., Hansen S., Holmskov U., Takahashi K., Stahl G.L., Dudler T., Girija U.V., Wallis R., Kadioglu A., Stover C.M., Andrew P.W., Schwaeble W.J. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8:e1002793. doi: 10.1371/journal.ppat.1002793. - DOI - PMC - PubMed
-
- Alves Pedroso M.L., Boldt a.B.W., Pereira-Ferrari L., Steffensen R., Strauss E., Jensenius J.C., Ioshii S.O., Messias-Reason I. Mannan-binding lectin MBL2 gene polymorphism in chronic hepatitis C: association with the severity of liver fibrosis and response to interferon therapy. Clin. Exp. Immunol. 2008;152:258–264. doi: 10.1111/j.1365-2249.2008.03614.x. - DOI - PMC - PubMed
-
- Ambrosio A.R., De Messias-Reason I.J.T. Leishmania (Viannia) braziliensis: interaction of mannose-binding lectin with surface glycoconjugates and complement activation. An antibody-independent defence mechanism. Parasite Immunol. 2005;27:333–340. doi: 10.1111/j.1365-3024.2005.00782.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

