IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients
- PMID: 25862561
- PMCID: PMC4447864
- DOI: 10.1182/blood-2014-12-615757
IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients
Abstract
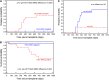
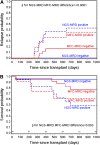
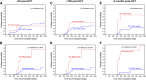
Positive detection of minimal residual disease (MRD) by multichannel flow cytometry (MFC) prior to hematopoietic cell transplantation (HCT) of patients with acute lymphoblastic leukemia (ALL) identifies patients at high risk for relapse, but many pre-HCT MFC-MRD negative patients also relapse, and the predictive power MFC-MRD early post-HCT is poor. To test whether the increased sensitivity of next-generation sequencing (NGS)-MRD better identifies pre- and post-HCT relapse risk, we performed immunoglobulin heavy chain (IgH) variable, diversity, and joining (V[D]J) DNA sequences J NGS-MRD on 56 patients with B-cell ALL enrolled in Children's Oncology Group trial ASCT0431. NGS-MRD predicted relapse and survival more accurately than MFC-MRD (P < .0001), especially in the MRD negative cohort (relapse, 0% vs 16%; P = .02; 2-year overall survival, 96% vs 77%; P = .003). Post-HCT NGS-MRD detection was better at predicting relapse than MFC-MRD (P < .0001), especially early after HCT (day 30 MFC-MRD positive relapse rate, 35%; NGS-MRD positive relapse rate, 67%; P = .004). Any post-HCT NGS positivity resulted in an increase in relapse risk by multivariate analysis (hazard ratio, 7.7; P = .05). Absence of detectable IgH-V(D)J NGS-MRD pre-HCT defines good-risk patients potentially eligible for less intense treatment approaches. Post-HCT NGS-MRD is highly predictive of relapse and survival, suggesting a role for this technique in defining patients early who would be eligible for post-HCT interventions. The trial was registered at www.clinicaltrials.gov as #NCT00382109.
© 2015 by The American Society of Hematology.
Figures
References
-
- Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. - PubMed
-
- Bader P, Kreyenberg H, Henze GH, et al. ALL-REZ BFM Study Group. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–384. - PubMed
-
- Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol. 2012;189(6):3221–3230. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

