Modified Ham test for atypical hemolytic uremic syndrome
- PMID: 25862562
- PMCID: PMC4784297
- DOI: 10.1182/blood-2015-02-629683
Modified Ham test for atypical hemolytic uremic syndrome
Abstract
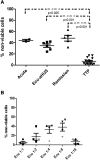
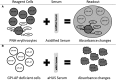
Atypical hemolytic uremic syndrome (aHUS) is a thrombotic microangiopathy (TMA) characterized by excessive activation of the alternative pathway of complement (APC). Atypical HUS is frequently a diagnosis of exclusion. Differentiating aHUS from other TMAs, especially thrombotic thrombocytopenic purpura (TTP), is difficult due to overlapping clinical manifestations. We sought to develop a novel assay to distinguish aHUS from other TMAs based on the hypothesis that paroxysmal nocturnal hemoglobinuria cells are more sensitive to APC-activated serum due to deficiency of glycosylphosphatidylinositol- anchored complement regulatory proteins (GPI-AP). Here, we demonstrate that phosphatidylinositol-specific phospholipase C-treated EA.hy926 cells and PIGA-mutant TF-1 cells are more susceptible to serum from aHUS patients than parental EA.hy926 and TF-1 cells. We next studied 31 samples from 25 patients with TMAs, including 9 with aHUS and 12 with TTP. Increased C5b-9 deposition was evident by confocal microscopy and flow cytometry on GPI-AP-deficient cells incubated with aHUS serum compared with heat-inactivated control, TTP, and normal serum. Differences in cell viability were observed in biochemically GPI-AP-deficient cells and were further increased in PIGA-deficient cells. Serum from patients with aHUS resulted in a significant increase of nonviable PIGA-deficient TF-1 cells compared with serum from healthy controls (P < .001) and other TMAs (P < .001). The cell viability assay showed high reproducibility, sensitivity, and specificity in detecting aHUS. In conclusion, we developed a simple, rapid, and serum-based assay that helps to differentiate aHUS from other TMAs.
© 2015 by The American Society of Hematology.
Figures
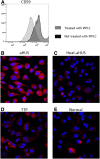
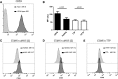
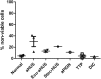
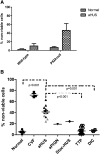
Comment in
-
Atypical HUS may become a diagnosis of inclusion.Blood. 2015 Jun 4;125(23):3525-6. doi: 10.1182/blood-2015-04-640656. Blood. 2015. PMID: 26045594
References
-
- Cataland SR, Wu HM. Diagnosis and management of complement mediated thrombotic microangiopathies. Blood Rev. 2014;28(2):67–74. - PubMed
-
- Nester CM, Thomas CP. Atypical hemolytic uremic syndrome: what is it, how is it diagnosed, and how is it treated? Hematology Am Soc Hematol Educ Program. 2012;2012:617-625. - PubMed
-
- Rennard S, Abe S. Decreased cold-insoluble globulin in congenital thrombocytopenia (Upshaw-Schulman syndrome). N Engl J Med. 1979;300(7):368. - PubMed
-
- Kinoshita S, Yoshioka A, Park YD, et al. Upshaw-Schulman syndrome revisited: a concept of congenital thrombotic thrombocytopenic purpura. Int J Hematol. 2001;74(1):101-108. - PubMed
-
- Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lammle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood. 1997;89(9):3097-3103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

