Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury
- PMID: 25862671
- PMCID: PMC4475464
- DOI: 10.1007/s10456-015-9465-6
Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury
Abstract
Rationale: Collaterals lessen tissue injury in occlusive disease. However, aging causes progressive decline in their number and smaller diameters in those that remain (collateral rarefaction), beginning at 16 months of age in mice (i.e., middle age), and worse ischemic injury-effects that are accelerated in even 3-month-old eNOS(-/-) mice. These findings have found indirect support in recent human studies.
Objective: We sought to determine whether other cardiovascular risk factors (CVRFs) associated with endothelial dysfunction cause collateral rarefaction, investigate possible mechanisms, and test strategies for prevention.
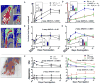
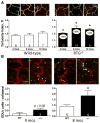
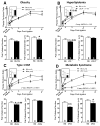
Methods and results: Mice with nine different models of CVRFs of 4-12 months of age were assessed for number and diameter of native collaterals in skeletal muscle and brain and for collateral-dependent perfusion and ischemic injury after arterial occlusion. Hypertension caused collateral rarefaction whose severity increased with duration and level of hypertension, accompanied by greater hindlimb ischemia and cerebral infarct volume. Chronic treatment of wild-type mice with L-N (G)-nitro-arginine methylester caused similar rarefaction and worse ischemic injury which were not prevented by lowering arterial pressure with hydralazine. Metabolic syndrome, hypercholesterolemia, diabetes mellitus, and obesity also caused collateral rarefaction. Neither chronic statin treatment nor exercise training lessened hypertension-induced rarefaction.
Conclusion: Chronic CVRF presence caused collateral rarefaction and worse ischemic injury, even at relatively young ages. Rarefaction was associated with increased proliferation rate of collateral endothelial cells, effects that may promote accelerated endothelial cell senescence.
Conflict of interest statement
Disclosure/Conflict of Interest: None for any of the authors
Figures
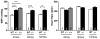
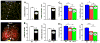


References
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:143–152. - PubMed
-
- Chin CT, Chen AY, Wang TY, Alexander KP, Mathews R, Rumsfeld JS, Cannon CP, Fonarow GC, Peterson ED, Roe MT. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: The acute coronary treatment and intervention outcomes network (action) registry-get with the guidelines (gwtg) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–122. - PubMed
-
- Koennecke HC, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, Kreitsch P, Mackert BM, Nabavi DG, Nolte CH, Pohls W, Schmehl I, Schmitz B, von Brevern M, Walter G, Heuschmann PU. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology. 2011;77:965–972. - PubMed
-
- Virkkunen J, Heikkinen M, Lepantalo M, Metsanoja R, Salenius JP. Diabetes as an independent risk factor for early postoperative complications in critical limb ischemia. J Vasc Surg. 2004;40:761–767. - PubMed
-
- Williams B, Menon M, Satran D, Hayward D, Hodges JS, Burke MN, Johnson RK, Poulose AK, Traverse JH, Henry TD. Patients with coronary artery disease not amenable to traditional revascularization: Prevalence and 3-year mortality. Catheter Cardiovasc Interv. 2010;75:886–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

