Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice
- PMID: 25862817
- PMCID: PMC4417391
- DOI: 10.4049/jimmunol.1402295
Obesity Increases Mortality and Modulates the Lung Metabolome during Pandemic H1N1 Influenza Virus Infection in Mice
Abstract
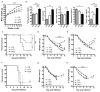
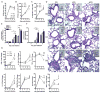
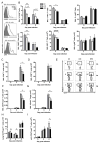
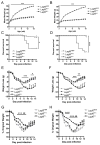
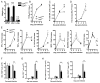
Obese individuals are at greater risk for hospitalization and death from infection with the 2009 pandemic H1N1 influenza virus (pH1N1). In this study, diet-induced and genetic-induced obese mouse models were used to uncover potential mechanisms by which obesity increases pH1N1 severity. High-fat diet-induced and genetic-induced obese mice exhibited greater pH1N1 mortality, lung inflammatory responses, and excess lung damage despite similar levels of viral burden compared with lean control mice. Furthermore, obese mice had fewer bronchoalveolar macrophages and regulatory T cells during infection. Obesity is inherently a metabolic disease, and metabolic profiling has found widespread usage in metabolic and infectious disease models for identifying biomarkers and enhancing understanding of complex mechanisms of disease. To further characterize the consequences of obesity on pH1N1 infection responses, we performed global liquid chromatography-mass spectrometry metabolic profiling of lung tissue and urine. A number of metabolites were perturbed by obesity both prior to and during infection. Uncovered metabolic signatures were used to identify changes in metabolic pathways that were differentially altered in the lungs of obese mice such as fatty acid, phospholipid, and nucleotide metabolism. Taken together, obesity induces distinct alterations in the lung metabolome, perhaps contributing to aberrant pH1N1 immune responses.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures



References
-
- Centers for Disease Control and Prevention. 2009 H1N1: Overview of a Pandemic. 2010 Dec; [cited 19 August 2014] http://www.cdc.gov/h1n1flu/yearinreview/yir1.html.
-
- World Health Organization. A (H1N1) seasonal influenza virus: guidance on prevention and treatment. 2014 Feb; [cited 19 August 2014] http://www.emro.who.int/egy/egypt-news/a-h1n1-is-a-seasonal-influenza-vi....
-
- Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT the California Pandemic (H1N1) Working Group . A Novel Risk Factor for a Novel Virus: Obesity and 2009 Pandemic Influenza A (H1N1) Clin Infect Dis. 2011;52:301–312. - PubMed
-
- Santa-Olalla Peralta P, Cortes-Garcia M, Vicente-Herrero M, Castrillo-Villamandos C, Arias-Bohigas P, Pachon-del Amo I, Sierra-Moros MJ Surveillance Group for New Influenza A(H1N1) Virus Investigation and Control Team in Spain . Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April - December 2009. Euro Surveill. 2010;15:19667. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

