Front-to-rear membrane tension gradient in rapidly moving cells
- PMID: 25863051
- PMCID: PMC4390806
- DOI: 10.1016/j.bpj.2015.02.007
Front-to-rear membrane tension gradient in rapidly moving cells
Abstract
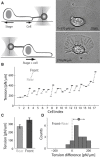
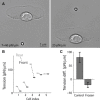
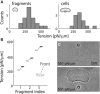
Membrane tension is becoming recognized as an important mechanical regulator of motile cell behavior. Although membrane-tension measurements have been performed in various cell types, the tension distribution along the plasma membrane of motile cells has been largely unexplored. Here, we present an experimental study of the distribution of tension in the plasma membrane of rapidly moving fish epithelial keratocytes. We find that during steady movement the apparent membrane tension is ∼30% higher at the leading edge than at the trailing edge. Similar tension differences between the front and the rear of the cell are found in keratocyte fragments that lack a cell body. This front-to-rear tension variation likely reflects a tension gradient developed in the plasma membrane along the direction of movement due to viscous friction between the membrane and the cytoskeleton-attached protein anchors embedded in the membrane matrix. Theoretical modeling allows us to estimate the area density of these membrane anchors. Overall, our results indicate that even though membrane tension equilibrates rapidly and mechanically couples local boundary dynamics over cellular scales, steady-state variations in tension can exist in the plasma membranes of moving cells.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Gauthier N.C., Masters T.A., Sheetz M.P. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22:527–535. - PubMed
-
- Lieber A.D., Yehudai-Resheff S., Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol. 2013;23:1409–1417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

