Calcium enhances binding of Aβ monomer to DMPC lipid bilayer
- PMID: 25863071
- PMCID: PMC4390832
- DOI: 10.1016/j.bpj.2015.03.001
Calcium enhances binding of Aβ monomer to DMPC lipid bilayer
Abstract
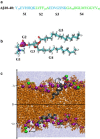
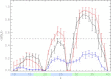
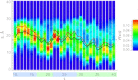
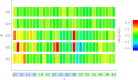
Using isobaric-isothermal replica-exchange molecular dynamics and the all-atom explicit-solvent model, we studied the equilibrium binding of Aβ monomers to a zwitterionic dimyristoylphosphatidylcholine (DMPC) bilayer coincubated with calcium ions. Using our previous replica-exchange molecular dynamics calcium-free simulations as a control, we reached three conclusions. First, calcium ions change the tertiary structure of the bound Aβ monomer by destabilizing several long-range intrapeptide interactions, particularly the salt bridge Asp(23)-Lys(28). Second, calcium strengthens Aβ peptide binding to the DMPC bilayer by enhancing electrostatic interactions between charged amino acids and lipid polar headgroups. As a result, Aβ monomer penetrates deeper into the bilayer, making disorder in proximal lipids and bilayer thinning more pronounced. Third, because calcium ions demonstrate strong affinity to negatively charged amino acids, a considerable influx of calcium into the area proximal to the bound Aβ monomer is observed. Consequently, the localizations of negatively charged amino acids and calcium ions in the Aβ binding footprint overlap. Based on our data, we propose a mechanism by which calcium ions strengthen Aβ-bilayer interactions. This mechanism involves two factors: 1) calcium ions make the DMPC bilayer partially cationic and thus attractive to the anionic Aβ peptide; and 2) destabilization of the Asp(23)-Lys(28) salt bridge makes Lys(28) available for interactions with the bilayer. Finally, we conclude that a single Aβ monomer does not promote permeation of calcium ions through the zwitterionic bilayer.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
The Alzheimer's disease Aβ peptide binds to the anionic DMPS lipid bilayer.Biochim Biophys Acta. 2016 Jun;1858(6):1118-28. doi: 10.1016/j.bbamem.2016.03.001. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26947182
-
Cholesterol Changes the Mechanisms of Aβ Peptide Binding to the DMPC Bilayer.J Chem Inf Model. 2017 Oct 23;57(10):2554-2565. doi: 10.1021/acs.jcim.7b00431. Epub 2017 Oct 2. J Chem Inf Model. 2017. PMID: 28910085
-
Binding of Aβ peptide creates lipid density depression in DMPC bilayer.Biochim Biophys Acta. 2014 Oct;1838(10):2678-88. doi: 10.1016/j.bbamem.2014.07.010. Epub 2014 Jul 15. Biochim Biophys Acta. 2014. PMID: 25037005
-
Inclusion of lipopeptides into the DMPC lipid bilayers prevents Aβ peptide insertion.Phys Chem Chem Phys. 2017 Apr 12;19(15):10087-10098. doi: 10.1039/c7cp01003f. Phys Chem Chem Phys. 2017. PMID: 28367578
-
Alzheimer's Aβ10-40 peptide binds and penetrates DMPC bilayer: an isobaric-isothermal replica exchange molecular dynamics study.J Phys Chem B. 2014 Mar 13;118(10):2638-48. doi: 10.1021/jp412153s. Epub 2014 Feb 28. J Phys Chem B. 2014. PMID: 24547901
Cited by
-
Is the Conformational Ensemble of Alzheimer's Aβ10-40 Peptide Force Field Dependent?PLoS Comput Biol. 2017 Jan 13;13(1):e1005314. doi: 10.1371/journal.pcbi.1005314. eCollection 2017 Jan. PLoS Comput Biol. 2017. PMID: 28085875 Free PMC article.
-
The Mechanism of Action of SAAP-148 Antimicrobial Peptide as Studied with NMR and Molecular Dynamics Simulations.Pharmaceutics. 2023 Feb 24;15(3):761. doi: 10.3390/pharmaceutics15030761. Pharmaceutics. 2023. PMID: 36986623 Free PMC article.
-
An Overview of Several Inhibitors for Alzheimer's Disease: Characterization and Failure.Int J Mol Sci. 2021 Oct 6;22(19):10798. doi: 10.3390/ijms221910798. Int J Mol Sci. 2021. PMID: 34639140 Free PMC article. Review.
-
Calcium inhibits penetration of Alzheimer's Aβ1 -42 monomers into the membrane.Proteins. 2022 Dec;90(12):2124-2143. doi: 10.1002/prot.26403. Epub 2022 Aug 10. Proteins. 2022. PMID: 36321654 Free PMC article.
-
Monomeric Amyloid Peptide-induced Toxicity in Human Oligodendrocyte Cell Line and Mouse Brain Primary Mixed-glial Cell Cultures: Evidence for a Neuroprotective Effect of Neurosteroid 3α-O-allyl-allopregnanolone.Neurotox Res. 2024 Aug 5;42(4):37. doi: 10.1007/s12640-024-00715-1. Neurotox Res. 2024. PMID: 39102123
References
-
- Haass C., Schlossmacher M.G., Selkoe D.J. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. - PubMed
-
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. - PubMed
-
- Williams T.L., Serpell L.C. Membrane and surface interactions of Alzheimer’s Aβ peptide—insights into the mechanism of cytotoxicity. FEBS J. 2011;278:3905–3917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

