A decision support framework for genomically informed investigational cancer therapy
- PMID: 25863335
- PMCID: PMC4651038
- DOI: 10.1093/jnci/djv098
A decision support framework for genomically informed investigational cancer therapy
Abstract
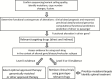
Rapidly improving understanding of molecular oncology, emerging novel therapeutics, and increasingly available and affordable next-generation sequencing have created an opportunity for delivering genomically informed personalized cancer therapy. However, to implement genomically informed therapy requires that a clinician interpret the patient's molecular profile, including molecular characterization of the tumor and the patient's germline DNA. In this Commentary, we review existing data and tools for precision oncology and present a framework for reviewing the available biomedical literature on therapeutic implications of genomic alterations. Genomic alterations, including mutations, insertions/deletions, fusions, and copy number changes, need to be curated in terms of the likelihood that they alter the function of a "cancer gene" at the level of a specific variant in order to discriminate so-called "drivers" from "passengers." Alterations that are targetable either directly or indirectly with approved or investigational therapies are potentially "actionable." At this time, evidence linking predictive biomarkers to therapies is strong for only a few genomic markers in the context of specific cancer types. For these genomic alterations in other diseases and for other genomic alterations, the clinical data are either absent or insufficient to support routine clinical implementation of biomarker-based therapy. However, there is great interest in optimally matching patients to early-phase clinical trials. Thus, we need accessible, comprehensive, and frequently updated knowledge bases that describe genomic changes and their clinical implications, as well as continued education of clinicians and patients.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Genomically guided cancer treatments: from "promising" to "clinically useful".J Natl Cancer Inst. 2015 May 28;107(7):djv168. doi: 10.1093/jnci/djv168. Print 2015 Jul. J Natl Cancer Inst. 2015. PMID: 26023091 No abstract available.
References
-
- Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28:Abstract No.3534.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources