Networking in the nucleus: a spotlight on LEM-domain proteins
- PMID: 25863918
- PMCID: PMC4522374
- DOI: 10.1016/j.ceb.2015.03.005
Networking in the nucleus: a spotlight on LEM-domain proteins
Abstract
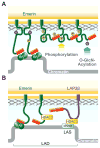
Proteins resident in the inner nuclear membrane and underlying nuclear lamina form a network that regulates nuclear functions. This review highlights a prominent family of nuclear lamina proteins that carries the LAP2-emerin-MAN1-domain (LEM-D). LEM-D proteins share an ability to bind lamins and tether repressive chromatin at the nuclear periphery. The importance of this family is underscored by findings that loss of individual LEM-D proteins causes progressive, tissue-restricted diseases, known as laminopathies. Diverse functions of LEM-D proteins are linked to interactions with unique and overlapping partners including signal transduction effectors, transcription factors and architectural proteins. Recent investigations suggest that LEM-D proteins form hubs within the nuclear lamina that integrate external signals important for tissue homeostasis and maintenance of progenitor cell populations.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

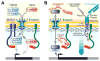
References
-
- Shimi T, et al. Nuclear lamins in cell regulation and disease. Cold Spring Harb Symp Quant Biol. 2010;75:525–31. - PubMed
-
- Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci. 2005;30(10):551–8. - PubMed
-
- Korfali N, et al. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3(6):552–64. This study demonstrated the extensive tissue-specificity of nuclear membrane protein expression, with tissue differences predicted to impact developmental signaling cascades and gene regulation. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

