Hydrogen sulfide and polysulfides as signaling molecules
- PMID: 25864468
- PMCID: PMC4568289
- DOI: 10.2183/pjab.91.131
Hydrogen sulfide and polysulfides as signaling molecules
Abstract
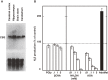
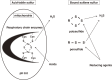
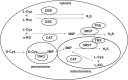
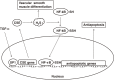
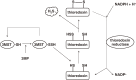
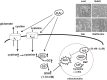
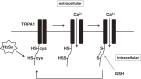
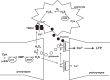
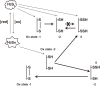
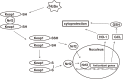
Hydrogen sulfide (H2S) is a familiar toxic gas that smells of rotten eggs. After the identification of endogenous H2S in the mammalian brain two decades ago, studies of this molecule uncovered physiological roles in processes such as neuromodulation, vascular tone regulation, cytoprotection against oxidative stress, angiogenesis, anti-inflammation, and oxygen sensing. Enzymes that produce H2S, such as cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase have been studied intensively and well characterized. Polysulfides, which have a higher number of inner sulfur atoms than that in H2S, were recently identified as potential signaling molecules that can activate ion channels, transcription factors, and tumor suppressors with greater potency than that of H2S. This article focuses on our contribution to the discovery of these molecules and their metabolic pathways and mechanisms of action.
Figures



References
-
- Furchgott R.F., Zawadzki J.V. (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376. - PubMed
-
- Furchgott, R.F. (1988) Studies on relaxation of rabbit aorta by sodium nitrate: basis for the proposal that the acid-activatable component of the inhibitory factor from retractor penis is inorganic nitrate and the endothelium-derived relaxing factor is nitric oxide. In Mechanisms of Vasodilatation (ed. Vanhoutte, P.M.). Raven, New York, pp. 401–414.
-
- Ignarro, L.J., Byrns, R.E. and Wood, K.S. (1988) Biochemical and pharmacological properties of endothelium-derived relaxing factor and its similarity to nitric oxide radical. In Mechanisms of Vasodilatation (ed. Vanhoutte, P.M.). Raven, New York, pp. 427–435.
-
- Garthwaite J., Charles S.L., Chess-Williams R. (1988) Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

