Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways
- PMID: 25864648
- PMCID: PMC4594189
- DOI: 10.1038/aps.2014.130
Fructus phyllanthi tannin fraction induces apoptosis and inhibits migration and invasion of human lung squamous carcinoma cells in vitro via MAPK/MMP pathways
Abstract
Aim: Fructus phyllanthi tannin fraction (PTF) from the traditional Tibetan medicine Fructus phyllanthi has been found to inhibit lung and liver carcinoma in mice. In this study we investigated the anticancer mechanisms of PTF in human lung squamous carcinoma cells in vitro.
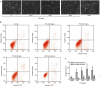
Methods: Human lung squamous carcinoma cell line (NCI-H1703), human large-cell lung cancer cell line (NCI-H460), human lung adenocarcinoma cell line (A549) and human fibrosarcoma cell line (HT1080) were tested. Cell viability was detected with MTT assay. Cell migration and invasion were assessed using a wound healing assay and a transwell chemotaxis chambers assay, respectively. Cell apoptosis was analyzed with flow cytometric analysis. The levels of apoptosis-related and metastasis-related proteins were detected by Western blot and immunofluorescence.
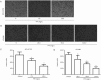
Results: PTF dose-dependently inhibited the viability of the 3 human lung cancer cells. The IC50 values of PTF in inhibition of NCI-H1703, NCI-H460, and A549 cells were 33, 203, and 94 mg/L, respectively. PTF (15, 30, and 60 mg/L) dose-dependently induced apoptosis of NCI-H1703 cells. Treatment of NCI-H1703 and HT1080 cells with PTF significantly inhibited cell migration, and reduced the number of invasive cells through Matrigel. Furthermore, PTF dose-dependently down-regulated the expression of phosphor-ERK1/2, MMP-2 and MMP-9, up-regulated the expression of phosphor-JNK, but had no significant effect on the expression of ERK1/2 or JNK.
Conclusion: PTF induces cell apoptosis and inhibits the migration and invasion of NCI-H1703 cells by decreasing MPPs expression through regulation of the MAPK pathway.
Figures


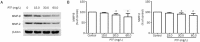
Similar articles
-
Curcumin induces apoptosis and suppresses invasion through MAPK and MMP signaling in human monocytic leukemia SHI-1 cells.Pharm Biol. 2016 Aug;54(8):1303-11. doi: 10.3109/13880209.2015.1060508. Epub 2015 Jul 1. Pharm Biol. 2016. PMID: 26134921
-
Dihydromyricetin inhibits migration and invasion of hepatoma cells through regulation of MMP-9 expression.World J Gastroenterol. 2014 Aug 7;20(29):10082-93. doi: 10.3748/wjg.v20.i29.10082. World J Gastroenterol. 2014. PMID: 25110435 Free PMC article.
-
Bufalin Inhibits NCI-H460 Human Lung Cancer Cell Metastasis In Vitro by Inhibiting MAPKs, MMPs, and NF-κB Pathways.Am J Chin Med. 2015;43(6):1247-64. doi: 10.1142/S0192415X15500718. Am J Chin Med. 2015. PMID: 26446205
-
Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer.World J Gastroenterol. 2015 Nov 7;21(41):11673-9. doi: 10.3748/wjg.v21.i41.11673. World J Gastroenterol. 2015. PMID: 26556994 Free PMC article. Review.
-
Curcumin May Prevent Basement Membrane Disassembly by Matrix Metalloproteinases and Progression of the Bladder Cancer.Nutrients. 2021 Dec 23;14(1):32. doi: 10.3390/nu14010032. Nutrients. 2021. PMID: 35010907 Free PMC article. Review.
Cited by
-
Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment.Int J Mol Sci. 2021 Oct 7;22(19):10827. doi: 10.3390/ijms221910827. Int J Mol Sci. 2021. PMID: 34639167 Free PMC article. Review.
-
Fangjiomics: revealing adaptive omics pharmacological mechanisms of the myriad combination therapies to achieve personalized medicine.Acta Pharmacol Sin. 2015 Jun;36(6):651-3. doi: 10.1038/aps.2015.33. Acta Pharmacol Sin. 2015. PMID: 26036241 Free PMC article. No abstract available.
-
Mebendazole augments sensitivity to sorafenib by targeting MAPK and BCL-2 signalling in n-nitrosodiethylamine-induced murine hepatocellular carcinoma.Sci Rep. 2019 Dec 13;9(1):19095. doi: 10.1038/s41598-019-55666-x. Sci Rep. 2019. PMID: 31836811 Free PMC article.
-
Comprehensive Genomic Characterization Analysis Identifies an Oncogenic Pseudogene RP11-3543B.1 in Human Gastric Cancer.Front Cell Dev Biol. 2021 Sep 29;9:743652. doi: 10.3389/fcell.2021.743652. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34660601 Free PMC article.
-
Phytochemical Analysis Using UPLC-MSn Combined with Network Pharmacology Approaches to Explore the Biomarkers for the Quality Control of the Anticancer Tannin Fraction of Phyllanthus emblica L. Habitat in Nepal.Evid Based Complement Alternat Med. 2021 Mar 25;2021:6623791. doi: 10.1155/2021/6623791. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33833816 Free PMC article.
References
-
- 4Huo XS. The expression of CD44s, CD44v6, MMP-2, MMP-9, TIMP-1 and TIMP-2 and their significance in non-small cell lung cancer [dissertation]. Tianjin: Tianjin Medical University; 2006.
-
- 5Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett 2013; 328: 207–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

