N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3
- PMID: 25864722
- PMCID: PMC4472561
- DOI: 10.1111/febs.13294
N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3
Abstract
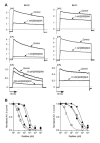
The voltage-gated potassium channel Kv1.3 is an important target for the treatment of autoimmune diseases and asthma. Blockade of Kv1.3 by the sea anemone peptide K⁺-channel toxin from Stichodactyla helianthus (ShK) inhibits the proliferation of effector memory T lymphocytes and ameliorates autoimmune diseases in animal models. However, the lack of selectivity of ShK for Kv1.3 over the Kv1.1 subtype has driven a search for Kv1.3-selective analogues. In the present study, we describe N-terminally extended analogues of ShK that contain a negatively-charged Glu, designed to mimic the phosphonate adduct in earlier Kv1.3-selective analogues, and consist entirely of common protein amino acids. Molecular dynamics simulations indicated that a Trp residue at position [-3] of the tetrapeptide extension could form stable interactions with Pro377 of Kv1.3 and best discriminates between Kv1.3 and Kv1.1. This led to the development of ShK with an N-terminal Glu-Trp-Ser-Ser extension ([EWSS]ShK), which inhibits Kv1.3 with an IC₅₀ of 34 pm and is 158-fold selective for Kv1.3 over Kv1.1. In addition, [EWSS]ShK is more than 2900-fold more selective for Kv1.3 over Kv1.2 and KCa3.1 channels. As a highly Kv1.3-selective analogue of ShK based entirely on protein amino acids, which can be produced by recombinant expression, this peptide is a valuable addition to the complement of therapeutic candidates for the treatment of autoimmune diseases.
Keywords: N-terminal extension; ShK; electrophysiology; molecular dynamics; potassium channels.
© 2015 FEBS.
Conflict of interest statement
Conflict of interest: CB and MWP are inventors on the patent (WO2006042151A2) describing ShK-186 and analogues. Kineta Inc. (Seattle, WA. USA) has licensed this patent from the University of California and is developing this peptide as a therapeutic for treatment of autoimmune diseases. CB is a consultant to Kineta Inc. The other authors declare no conflict of interest.
Figures



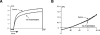
Similar articles
-
A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3.FEBS Lett. 2012 Nov 16;586(22):3996-4001. doi: 10.1016/j.febslet.2012.09.038. Epub 2012 Oct 9. FEBS Lett. 2012. PMID: 23063513 Free PMC article.
-
Role of disulfide bonds in the structure and potassium channel blocking activity of ShK toxin.Biochemistry. 1999 Nov 2;38(44):14549-58. doi: 10.1021/bi991282m. Biochemistry. 1999. PMID: 10545177
-
Designed peptide analogues of the potassium channel blocker ShK toxin.Biochemistry. 2001 Dec 25;40(51):15528-37. doi: 10.1021/bi011300b. Biochemistry. 2001. PMID: 11747428
-
Potassium channel blockade by the sea anemone toxin ShK for the treatment of multiple sclerosis and other autoimmune diseases.Curr Med Chem. 2004 Dec;11(23):3041-52. doi: 10.2174/0929867043363947. Curr Med Chem. 2004. PMID: 15578998 Review.
-
Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases.Inflamm Allergy Drug Targets. 2011 Oct;10(5):313-21. doi: 10.2174/187152811797200641. Inflamm Allergy Drug Targets. 2011. PMID: 21824083 Free PMC article. Review.
Cited by
-
Optimization of Pichia pastoris Expression System for High-Level Production of Margatoxin.Front Pharmacol. 2021 Sep 29;12:733610. doi: 10.3389/fphar.2021.733610. eCollection 2021. Front Pharmacol. 2021. PMID: 34658872 Free PMC article.
-
Proteotransciptomics of the Most Popular Host Sea Anemone Entacmaea quadricolor Reveals Not All Toxin Genes Expressed by Tentacles Are Recruited into Its Venom Arsenal.Toxins (Basel). 2024 Feb 5;16(2):85. doi: 10.3390/toxins16020085. Toxins (Basel). 2024. PMID: 38393163 Free PMC article.
-
The antifungal plant defensin AtPDF2.3 from Arabidopsis thaliana blocks potassium channels.Sci Rep. 2016 Aug 30;6:32121. doi: 10.1038/srep32121. Sci Rep. 2016. PMID: 27573545 Free PMC article.
-
Study of Small-Molecule-Membrane Protein Binding Kinetics with Nanodisc and Charge-Sensitive Optical Detection.Anal Chem. 2016 Feb 16;88(4):2375-9. doi: 10.1021/acs.analchem.5b04366. Epub 2016 Jan 25. Anal Chem. 2016. PMID: 26752355 Free PMC article.
-
Structure of the voltage-gated potassium channel KV1.3: Insights into the inactivated conformation and binding to therapeutic leads.Channels (Austin). 2023 Dec;17(1):2253104. doi: 10.1080/19336950.2023.2253104. Channels (Austin). 2023. PMID: 37695839 Free PMC article. Review.
References
-
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168–J177. - PubMed
-
- Moroni L, Bianchi I, Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun Rev. 2012;11:A386–A392. - PubMed
-
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. - PubMed
-
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

