Oncometabolites: tailoring our genes
- PMID: 25864878
- PMCID: PMC4676302
- DOI: 10.1111/febs.13295
Oncometabolites: tailoring our genes
Abstract
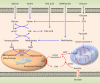
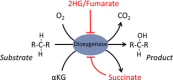
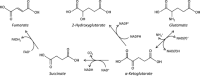
Increased glucose metabolism in cancer cells is a phenomenon that has been known for over 90 years, allowing maximal cell growth through faster ATP production and redistribution of carbons towards nucleotide, protein and fatty acid synthesis. Recently, metabolites that can promote tumorigeneis by altering the epigenome have been identified. These 'oncometabolites' include the tricarboxylic acid cycle metabolites succinate and fumarate, whose levels are elevated in rare tumours with succinate dehydrogenase and fumarate hydratase mutations, respectively. 2-Hydroxyglutarate is another oncometabolite; it is produced de novo as a result of the mutation of isocitrate dehydrogenase, and is commonly found in gliomas and acute myeloid leukaemia. Interestingly, the structural similarity of these oncometabolites to their precursor metabolite, α-ketoglutarate, explains the tumorigenic potential of these metabolites, by competitive inhibition of a superfamily of enzymes called the α-ketoglutarate-dependent dioxygenases. These enzymes utilize α-ketoglutarate as a cosubstrate, and are involved in fatty acid metabolism, oxygen sensing, collagen biosynthesis, and modulation of the epigenome. They include enzymes that are involved in regulating gene expression via DNA and histone tail demethylation. In this review, we will focus on the link between metabolism and epigenetics, and how we may target oncometabolite-induced tumorigenesis in the future.
Keywords: cancer; dioxygenase; epigenetics; metabolism; oncometabolites.
© 2015 The Authors. FEBS Journal published by John Wiley & Sons Ltd on behalf of FEBS.
Figures
References
-
- Warburg O (1956) On the origin of cancer cells. Science 123, 309–314. - PubMed
-
- Ben‐Haim S & Ell P (2009) 18F‐FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med 50, 88–99. - PubMed
-
- Higashi K, Ueda Y, Yagishita M, Arisaka Y, Sakurai A, Oguchi M, Seki H, Nambu Y, Tonami H & Yamamoto I (2000) FDG PET measurement of the proliferative potential of non‐small cell lung cancer. J Nucl Med 41, 85–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

