Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test
- PMID: 25865152
- PMCID: PMC4443858
- DOI: 10.1016/j.bbr.2015.04.002
Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test
Abstract
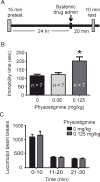
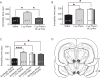
Recent studies revealed a causal link between ventral tegmental area (VTA) phasic dopamine (DA) activity and pro-depressive and antidepressant-like behavioral responses in rodent models of depression. Cholinergic activity in the VTA has been demonstrated to regulate phasic DA activity, but the role of VTA cholinergic mechanisms in depression-related behavior is unclear. The goal of this study was to determine whether pharmacological manipulation of VTA cholinergic activity altered behavioral responding in the forced swim test (FST) in rats. Here, male Sprague-Dawley rats received systemic or VTA-specific administration of the acetylcholinesterase inhibitor, physostigmine (systemic; 0.06 or 0.125mg/kg, intra-cranial; 1 or 2μg/side), the muscarinic acetylcholine receptor (AChR) antagonist scopolamine (2.4 or 24μg/side), or the nicotinic AChR antagonist mecamylamine (3 or 30μg/side), prior to the FST test session. In control experiments, locomotor activity was also examined following systemic and intra-cranial administration of cholinergic drugs. Physostigmine administration, either systemically or directly into the VTA, significantly increased immobility time in FST, whereas physostigmine infusion into a dorsal control site did not alter immobility time. In contrast, VTA infusion of either scopolamine or mecamylamine decreased immobility time, consistent with an antidepressant-like effect. Finally, the VTA physostigmine-induced increase in immobility was blocked by co-administration with scopolamine, but unaltered by co-administration with mecamylamine. These data show that enhancing VTA cholinergic tone and blocking VTA AChRs has opposing effects in FST. Together, the findings provide evidence for a role of VTA cholinergic mechanisms in behavioral responses in FST.
Keywords: Acetylcholine; Forced swim test; Mecamylamine; Physostigmine; Scopolamine; Ventral tegmental area.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures



References
-
- Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–105. - PubMed
-
- Bella R, et al. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56(3):298–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

