Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes
- PMID: 25865256
- PMCID: PMC4394166
- DOI: 10.3402/jev.v4.26334
Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes
Abstract
The Epstein-Barr virus (EBV)-encoded oncoprotein latent membrane protein 1 (LMP1) constitutively activates nuclear factor κB (NFκB) from intracellular membranes to promote cell growth and survival. LMP1 associates with CD63 in intracellular membranes and is released via exosomes. Whether tumour necrosis factor (TNF) receptor-associated factors (TRAFs) mediate LMP1 NFκB signalling from endosomes and modulate exosomal sorting is unknown. In this article, we show that LMP1-TRAF2 signalling complexes accumulate at endosomes in a palmitoylation-dependent manner, thereby driving LMP1-dependent oncogenicity. Palmitoylation is a reversible post-translational modification and is considered to function as a membrane anchor for proteins. Mutagenesis studies showed that LMP1-TRAF2 trafficking to endosomes is dependent on one single cysteine residue (C78), a known palmitoylation site of LMP1. Notably, growth assays in soft agar revealed that oncogenic properties of the palmitoylation-deficient LMP1 mutant C78A were diminished compared to wild-type LMP1. Since LMP1 recruitment of TRAF2 and downstream NFκB signalling were not affected by a disturbance in palmitoylation, the specific localization of LMP1 at endosomal membranes appears crucial for its transforming potential. The importance of palmitoylation for trafficking to and signalling from endosomal membranes was not restricted to LMP1, as similar observations were made for the cellular oncoproteins Src and Fyn. Despite abundant LMP1-TRAF2 association at endosomal membranes TRAF2 could not be detected in exosomes by Western blotting or proteomics. Interestingly, point mutations that prevented TRAF binding strongly promoted the sorting and release of LMP1 via exosomes. These observations reveal that LMP1-TRAF2 complexes at endosomes support oncogenic NFκB activation and suggest that LMP1 dissociates from the activated signalling complexes upon sorting into intraluminal vesicles. We propose that "signalling endosomes" in EBV-infected tumour cells can fuse with the plasma membrane, explaining LMP1 release via exosomes.
Keywords: LMP1; endosomes; exosomes; oncogenic signalling; palmitoylation.
Figures
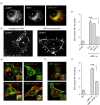

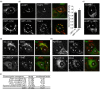
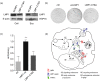
References
-
- Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–73. - PubMed
-
- Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011;13:827–37. - PubMed
-
- "/>Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. - PubMed
-
- Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008;18:322–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

