Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung
- PMID: 25865356
- PMCID: PMC4396689
- DOI: 10.1038/ncomms7727
Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung
Abstract
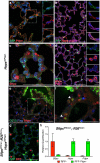
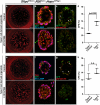
The plasticity of differentiated cells in adult tissues undergoing repair is an area of intense research. Pulmonary alveolar type II cells produce surfactant and function as progenitors in the adult, demonstrating both self-renewal and differentiation into gas exchanging type I cells. In vivo, type I cells are thought to be terminally differentiated and their ability to give rise to alternate lineages has not been reported. Here we show that Hopx becomes restricted to type I cells during development. However, unexpectedly, lineage-labelled Hopx(+) cells both proliferate and generate type II cells during adult alveolar regrowth following partial pneumonectomy. In clonal 3D culture, single Hopx(+) type I cells generate organoids composed of type I and type II cells, a process modulated by TGFβ signalling. These findings demonstrate unanticipated plasticity of type I cells and a bidirectional lineage relationship between distinct differentiated alveolar epithelial cell types in vivo and in single-cell culture.
Figures





References
-
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. - PubMed
-
- Kaplan HP, Robinson FR, Kapanci Y, Weibel ER. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. 1969;20:94–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL122521/HL/NHLBI NIH HHS/United States
- U01HL110942,/HL/NHLBI NIH HHS/United States
- T32 HD040372/HD/NICHD NIH HHS/United States
- U01 HL100405/HL/NHLBI NIH HHS/United States
- K08HL119553-02/HL/NHLBI NIH HHS/United States
- UO1HL110967/HL/NHLBI NIH HHS/United States
- U01 HL110967/HL/NHLBI NIH HHS/United States
- U01 HL110942/HL/NHLBI NIH HHS/United States
- K08 HL119553/HL/NHLBI NIH HHS/United States
- K08HL122521-01/HL/NHLBI NIH HHS/United States
- R01HL071546/HL/NHLBI NIH HHS/United States
- U01 HL111018/HL/NHLBI NIH HHS/United States
- R01 HL071546/HL/NHLBI NIH HHS/United States
- UO1HL111018/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

