Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use
- PMID: 25865392
- PMCID: PMC4516635
- DOI: 10.1016/j.joca.2015.03.036
Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use
Abstract
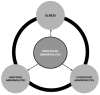
Osteoarthritis (OA) is a heterogeneous disorder. The goals of this review are (1) To stimulate use of standardized nomenclature for OA that could serve as building blocks for describing OA and defining OA phenotypes, in short to provide unifying disease concepts for a heterogeneous disorder; and (2) To stimulate establishment of ROAD (Risk of OA Development) and ROAP (Risk of OA Progression) tools analogous to the FRAX™ instrument for predicting risk of fracture in osteoporosis; and (3) To stimulate formulation of tools for identifying disease in its early preradiographic and/or molecular stages - REDI (Reliable Early Disease Identification). Consensus around more sensitive and specific diagnostic criteria for OA could spur development of disease modifying therapies for this entity that has proved so recalcitrant to date. We fully acknowledge that as we move forward, we expect to develop more sophisticated definitions, terminology and tools.
Keywords: Anatomy; Biomarkers; Criteria; Definition; Osteoarthritis; Physiology.
Copyright © 2015 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Virginia Byers Kraus -- Has received salary support through NIH grants PO1 AR050245 and AG028716; lecture/consultancy fees from Merrimack Pharmaceuticals, Flexion Therapeutics, Bioiberica and Abbott. She is an Associate Editor of
Francisco J Blanco -- has received Grants (Clinical Trials, conferences, advisor and publications) from: Abbvie, Amgen, Bioiberica, Bristol Mayer, Celgene, Celltrion, Cellerix, Grunenthal, Gebro Pharma, Lilly, MSD, Merck Serono, Pfizer, Pierre-Fabra, Roche, Sanofi, Servier and UCB.
Martin Englund – has received honorarium for lectures in a course in clinical epidemiology from Pfizer and for a lecture in knee OA from Össur.
Morten Karsdal - is a full time employee of Nordic Bioscience, a company engaged in biomarker and medicinal product research and development.
Stefan Lohmander -- Relevant financial activities outside the submitted work include consultancy for Abbvie, Flexion, Galapagos, Medivir, MerckSerono, Teijin, Össur. Employment as Editor-in-Chief of Osteoarthritis and Cartilage.
Figures
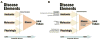

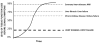
References
-
- Simoncell T, Barclay L, Bouri K, Burns K, Cook K, Filice R, et al. Paving the Way for Personalized Medicine: FDA’s Role in a New Era of Medical Product Development. Silver Spring, MD: Oct, 2013. pp. 1–61.
-
- Desmond-Hellmann S, Sawyers C, Cox D, Fraser-Liggett C, Galli S, Goldstein D, et al. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington DC: National Academy of Sciences; 2011. - PubMed
-
- Nordqvist C. Medical News Today: Medical News Today. 2014. What is physiology.
-
- Castaneda S, Roman-Blas JA, Largo R, Herrero-Beaumont G. Osteoarthritis: a progressive disease with changing phenotypes. Rheumatology (Oxford) 2014;53:1–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

