PTEN functions by recruitment to cytoplasmic vesicles
- PMID: 25866245
- PMCID: PMC4423730
- DOI: 10.1016/j.molcel.2015.03.011
PTEN functions by recruitment to cytoplasmic vesicles
Abstract
PTEN is proposed to function at the plasma membrane, where receptor tyrosine kinases are activated. However, the majority of PTEN is located throughout the cytoplasm. Here, we show that cytoplasmic PTEN is distributed along microtubules, tethered to vesicles via phosphatidylinositol 3-phosphate (PI(3)P), the signature lipid of endosomes. We demonstrate that the non-catalytic C2 domain of PTEN specifically binds PI(3)P through the CBR3 loop. Mutations render this loop incapable of PI(3)P binding and abrogate PTEN-mediated inhibition of PI 3-kinase/AKT signaling. This loss of function is rescued by fusion of the loop mutant PTEN to FYVE, the canonical PI(3)P binding domain, demonstrating the functional importance of targeting PTEN to endosomal membranes. Beyond revealing an upstream activation mechanism of PTEN, our data introduce the concept of PI 3-kinase signal activation on the vast plasma membrane that is contrasted by PTEN-mediated signal termination on the small, discrete surfaces of internalized vesicles.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
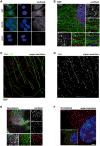


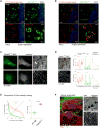
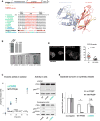
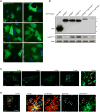
Comment in
-
Cancer: Precise control of localized signals.Nature. 2015 Jun 4;522(7554):38-40. doi: 10.1038/nature14531. Epub 2015 May 27. Nature. 2015. PMID: 26017306 No abstract available.
References
-
- Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. - PubMed
-
- Bravo J, Karathanassis D, Pacold CM, Pacold ME, Ellson CD, Anderson KE, Butler PJ, Lavenir I, Perisic O, Hawkins PT, et al. The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Mol Cell. 2001;8:829–839. - PubMed
-
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

