The Efficacy of Saccharomyces boulardii CNCM I-745 in Addition to Standard Helicobacter pylori Eradication Treatment in Children
- PMID: 25866729
- PMCID: PMC4391996
- DOI: 10.5223/pghn.2015.18.1.17
The Efficacy of Saccharomyces boulardii CNCM I-745 in Addition to Standard Helicobacter pylori Eradication Treatment in Children
Abstract
Purpose: This study aims to investigate Saccharomyces boulardii CNCM I-745 during Helicobacter pylori eradication in children.
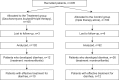
Methods: One hundred ninety-four H. pylori positive children were randomized in two groups. Therapy (omeprazole+clarithromycin+amoxicillin or omeprazole+clarithromycin+metronidazole in case of penicillin allergy) was given to both groups during two weeks. In the treatment group (n: 102) S. boulardii was added to the triple therapy, while the control group (n: 92) only received triple therapy. The incidence, onset, duration and severity of diarrhea and compliance to the eradication treatment were compared. A (13)C urea breath test was done 4 weeks after the end of eradication therapy in two groups of 21 patients aged 12 years and older to test the H. pylori eradication rate.
Results: In the treatment group, diarrhea occurred in 12 cases (11.76%), starting after 6.25±1.24 days, lasting 3.17±1.08 days, and compliance to eradication treatment was 100%. In the control group, diarrhea occurred in 26 cases (28.26%), starting after 4.05±1.11 days, lasting 4.02±0.87 days, and in six cases eradication treatment was stopped prematurely (p<0.05). The (13)C urea breath test showed successful H. pylori eradication in 71.4% of the patients in the treatment and in 61.9 % in the control group (not significant).
Conclusion: S. boulardii has a beneficial effect on the prevention and treatment of diarrhea during H. pylori eradication in children. Although S. boulardii did only slightly increase H. pylori eradication rate, compliance to eradication treatment was improved.
Keywords: Child; Compliance; Diarrhea; Helicobacter pylori; Prevention & control; Probiotics; Yeasts.
References
-
- Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami SQ. Helicobacter pylori infection in children: population-based age-specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99:279–282. - PubMed
-
- Yang XQ, Yi ZW. Pediatrics. 6th ed. Beijing: People's Medical Publishing House; 2003. pp. 295–296.
-
- Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. - PubMed
-
- Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, et al. H pylori Working Groups of ESPGHAN and NASPGHAN. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230–243. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources