TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts
- PMID: 25866970
- PMCID: PMC4463192
- DOI: 10.1172/JCI77321
TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts
Abstract
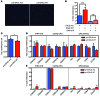
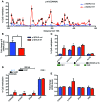
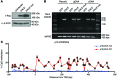
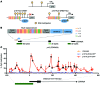
Current strategies to alter disease-associated epigenetic modifications target ubiquitously expressed epigenetic regulators. This approach does not allow specific genes to be controlled in specific cell types; therefore, tools to selectively target epigenetic modifications in the desired cell type and strategies to more efficiently correct aberrant gene expression in disease are needed. Here, we have developed a method for directing DNA methylation to specific gene loci by conjugating catalytic domains of DNA methyltransferases (DNMTs) to engineered transcription activator-like effectors (TALEs). We demonstrated that these TALE-DNMTs direct DNA methylation specifically to the targeted gene locus in human cells. Further, we determined that minimizing direct nucleotide sequence repeats within the TALE moiety permits efficient lentivirus transduction, allowing easy targeting of primary cell types. Finally, we demonstrated that directed DNA methylation with a TALE-DNMT targeting the CDKN2A locus, which encodes the cyclin-dependent kinase inhibitor p16, decreased CDKN2A expression and increased replication of primary human fibroblasts, as intended. Moreover, overexpression of p16 in these cells reversed the proliferative phenotype, demonstrating the specificity of our epigenetic targeting. Together, our results demonstrate that TALE-DNMTs can selectively target specific genes and suggest that this strategy has potential application for the development of locus-specific epigenetic therapeutics.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

