mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation
- PMID: 25867072
- PMCID: PMC4603992
- DOI: 10.1038/onc.2015.79
mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation
Abstract
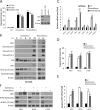
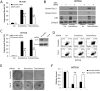
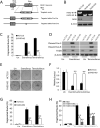
The mammalian target of rapamycin (mTOR) is commonly activated in colon cancer. mTOR complex 1 (mTORC1) is a major downstream target of the PI3K/ATK pathway and activates protein synthesis by phosphorylating key regulators of messenger RNA translation and ribosome synthesis. Rapamycin analogs Everolimus and Temsirolimus are non-ATP-competitive mTORC1 inhibitors, and suppress proliferation and tumor angiogenesis and invasion. We now show that apoptosis plays a key role in their anti-tumor activities in colon cancer cells and xenografts through the DR5, FADD and caspase-8 axis, and is strongly enhanced by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and 5-fluorouracil. The induction of DR5 by rapalogs is mediated by the ER stress regulator and transcription factor CHOP, but not the tumor suppressor p53, on rapid and sustained inhibition of 4E-BP1 phosphorylation, and attenuated by eIF4E expression. ATP-competitive mTOR/PI3K inhibitors also promote DR5 induction and FADD-dependent apoptosis in colon cancer cells. These results establish activation of ER stress and the death receptor pathway as a novel anticancer mechanism of mTOR inhibitors.
Figures

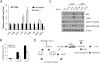

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

