Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study
- PMID: 25867139
- PMCID: PMC4520640
- DOI: 10.1111/cas.12674
Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC Study
Abstract
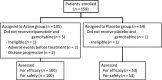
Gemcitabine is a key drug for the treatment of pancreatic cancer; however, with its limitation in clinical benefits, the development of another potent therapeutic is necessary. Vascular endothelial growth factor receptor 2 is an essential target for tumor angiogenesis, and we have conducted a phase I clinical trial using gemcitabine and vascular endothelial growth factor receptor 2 peptide (elpamotide). Based on the promising results of this phase I trial, a multicenter, randomized, placebo-controlled, double-blind phase II/III clinical trial has been carried out for pancreatic cancer. The eligibility criteria included locally advanced or metastatic pancreatic cancer. Patients were assigned to either the Active group (elpamotide + gemcitabine) or Placebo group (placebo + gemcitabine) in a 2:1 ratio by the dynamic allocation method. The primary endpoint was overall survival. The Harrington-Fleming test was applied to the statistical analysis in this study to evaluate the time-lagged effect of immunotherapy appropriately. A total of 153 patients (Active group, n = 100; Placebo group, n = 53) were included in the analysis. No statistically significant differences were found between the two groups in the prolongation of overall survival (Harrington-Fleming P-value, 0.918; log-rank P-value, 0.897; hazard ratio, 0.87, 95% confidence interval [CI], 0.486-1.557). Median survival time was 8.36 months (95% CI, 7.46-10.18) for the Active group and 8.54 months (95% CI, 7.33-10.84) for the Placebo group. The toxicity observed in both groups was manageable. Combination therapy of elpamotide with gemcitabine was well tolerated. Despite the lack of benefit in overall survival, subgroup analysis suggested that the patients who experienced severe injection site reaction, such as ulceration and erosion, might have better survival.
Keywords: Advanced pancreatic cancer; elpamotide; immunotherapy; peptide vaccine; phase II/III.
© 2015 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures


References
-
- Vital Statistics in Japan, tabulated by Center for Cancer Control and Information Services, National Cancer Center, Japan. [Webpage]. 2013/07/01 [cited 2013 September 19]; Available from URL: http://ganjoho.jp/pro/statistics/en/table_download.html.
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Monitoring of Cancer Incidence in Japan – Survival 2003–2005 Report (Center for Cancer Control and Information Services, National Cancer Center, 2013) [Japanese]. [Webpage]. 2013/07/01 [cited 2013 September 20]. Available from URL: http://ganjoho.jp/pro/statistics/en/table_download.html.
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–5. - PubMed
-
- Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

