TLR-3 is present in human adipocytes, but its signalling is not required for obesity-induced inflammation in adipose tissue in vivo
- PMID: 25867514
- PMCID: PMC4395029
- DOI: 10.1371/journal.pone.0123152
TLR-3 is present in human adipocytes, but its signalling is not required for obesity-induced inflammation in adipose tissue in vivo
Abstract
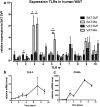
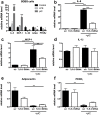
Innate immunity plays a pivotal role in obesity-induced low-grade inflammation originating from adipose tissue. Key receptors of the innate immune system including Toll-like receptors-2 and -4 (TLRs) are triggered by nutrient excess to promote inflammation. The role of other TLRs in this process is largely unknown. In addition to double-stranded viral mRNA, TLR-3 can also recognize mRNA from dying endogenous cells, a process that is frequently observed within obese adipose tissue. Here, we identified profound expression of TLR-3 in adipocytes and investigated its role during diet-induced obesity. Human adipose tissue biopsies (n=80) and an adipocyte cell-line were used to study TLR-3 expression and function. TLR-3-/- and WT animals were exposed to a high-fat diet (HFD) for 16 weeks to induce obesity. Expression of TLR-3 was significantly higher in human adipocytes compared to the non-adipocyte cells part of the adipose tissue. In vitro, TLR-3 expression was induced during differentiation of adipocytes and stimulation of the receptor led to elevated expression of pro-inflammatory cytokines. In vivo, TLR-3 deficiency did not significantly influence HFD-induced obesity, insulin sensitivity or inflammation. In humans, TLR-3 expression in adipose tissue did not correlate with BMI or insulin sensitivity (HOMA-IR). Together, our results demonstrate that TLR-3 is highly expressed in adipocytes and functionally active. However, TLR-3 appears to play a redundant role in obesity-induced inflammation and insulin resistance.
Conflict of interest statement
Figures



References
-
- WHO. WHO fact files: ten facts on obesity. 2010. Geneva: WHO.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

