A Simplified HAART Regimen with Raltegravir and Lamivudine, and Pharmacokinetic Interactions with a Combined Immunosuppressive Therapy with Tacrolimus and Everolimus in an HIV/HCV/HBV/HDV Patient after Liver Transplantation
- PMID: 25867565
- PMCID: PMC4668968
- DOI: 10.7727/wimj.2013.264
A Simplified HAART Regimen with Raltegravir and Lamivudine, and Pharmacokinetic Interactions with a Combined Immunosuppressive Therapy with Tacrolimus and Everolimus in an HIV/HCV/HBV/HDV Patient after Liver Transplantation
Abstract
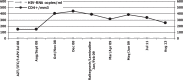
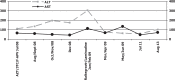
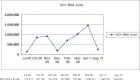
In this case report, we examine the impact of a simplified two-drug highly active antiretroviral therapy (HAART) regimen of raltegravir and lamivudine in a patient co-infected with human immunodeficiency virus (HIV) and hepatitis C, D and B viruses (HCV/HDV/HBV) under immunosuppressive therapy after liver transplantation. Pharmacokinetic interactions between integrase inhibitors and immunosuppressant drugs are described. Raltegravir, the first integrase inhibitor, associated with lamivudine, was introduced because its metabolism does not interfere with immunosuppressant therapy. During post-orthotopic liver transplantation follow-up, the patient's transaminases level increased and his antiretroviral therapy (HAART) of tenofovir/emtricitabine and fosamprenavir was changed, due to suspected drug toxicity. After seven months of follow-up, the patient showed good tolerance, good viro-immunological control with undetectable HIV viraemia and stable concentrations of immunosuppressive drugs. This case indicates that the combination of raltegravir and lamivudine is an optimal and effective strategy because it resulted in an important reduction of hepatic transaminases in a patient with very critical clinical conditions.
En este reporte de caso, examinamos el impacto de un régimen de terapia antirretroviral de gran actividad (TARGA) simplificada con dos drogas – raltegravir y lamivudina – en un paciente coinfectado con el virus de inmunodeficiencia humana (VIH) y los virus de la hepatitis C, D y B (HCV/HDV/HBV) bajo terapia inmunosupresiva después del trasplante del hígado. Se describen interacciones farmacocinéticas entre los inhibidores de la integrasa y los medicamentos inmunosupresores. El raltegravir – el primer inhibidor de la integrasa, asociado a la lamivudina – se introdujo porque su metabolismo no interfiere con la terapia inmunosupresora. Durante el seguimiento del trasplante hepático post-ortotópico del hígado, se produjo un aumento del nivel de transaminasas del paciente, y fue necesario cambiar la terapia antirretroviral de gran actividad (TARGA) con tenofovir-emtricitabina y fosamprenavir, debido a sospecha de toxicidad de los fármacos. Tras siete meses de seguimiento, el paciente mostró buena tolerancia, buen control viro-inmunológico con viremia del VIH indetectable y concentraciones estables de las medicamentos inmunosupresivos. Este caso indica que la combinación de raltegravir y lamivudina es una estrategia óptima y eficaz, ya que trajo consigo una reducción importante de las transaminasas hepáticas en un paciente con condiciones clínicas muy críticas.
Figures

References
-
- Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, et al. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection. Results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. - PubMed
-
- Moreno A, Quereda C, Moreno L, Pérez-Elías MJ, Muriel A, Casado JL, et al. Safe use of raltegravir and serolimus in an HIV-1 infected patient with renal impairment after orthotopic transplantation. AIDS. 2008;22:547–548. - PubMed
-
- Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. - PubMed
-
- Hassane I, Vacher VL, Baumelo A, Deray G. Antiretroviral and immunosuppressive drug-drug interactions: an update. Kidney Int. 2004;66:532–541. - PubMed
-
- Tricot L, Teicher E, Peytavin G, Zucman D, Conti F, Calmus Y, et al. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant. 2009;9:1946–1952. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

