Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes
- PMID: 25867762
- PMCID: PMC4447567
- DOI: 10.1038/labinvest.2015.51
Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes
Erratum in
-
Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes.Lab Invest. 2015 Oct;95(10):1218. doi: 10.1038/labinvest.2015.99. Lab Invest. 2015. PMID: 26412498 No abstract available.
Abstract
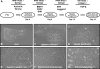
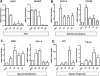
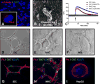
Cholangiocytes are the target of a heterogeneous group of liver diseases known as the cholangiopathies. An evolving understanding of the mechanisms driving biliary development provides the theoretical underpinnings for rational development of induced pluripotent stem cell (iPSC)-derived cholangiocytes (iDCs). Therefore, the aims of this study were to develop an approach to generate iDCs and to fully characterize the cells in vitro and in vivo. Human iPSC lines were generated by forced expression of the Yamanaka pluripotency factors. We then pursued a stepwise differentiation strategy toward iDCs, using precise temporal exposure to key biliary morphogens, and we characterized the cells, using a variety of morphologic, molecular, cell biologic, functional, and in vivo approaches. Morphology shows a stepwise phenotypic change toward an epithelial monolayer. Molecular analysis during differentiation shows appropriate enrichment in markers of iPSC, definitive endoderm, hepatic specification, hepatic progenitors, and ultimately cholangiocytes. Immunostaining, western blotting, and flow cytometry demonstrate enrichment of multiple functionally relevant biliary proteins. RNA sequencing reveals that the transcriptome moves progressively toward that of human cholangiocytes. iDCs generate intracellular calcium signaling in response to ATP, form intact primary cilia, and self-assemble into duct-like structures in three-dimensional culture. In vivo, the cells engraft within mouse liver, following retrograde intrabiliary infusion. In summary, we have developed a novel approach to generate mature cholangiocytes from iPSCs. In addition to providing a model of biliary differentiation, iDCs represent a platform for in vitro disease modeling, pharmacologic testing, and individualized, cell-based, regenerative therapies for the cholangiopathies.
Conflict of interest statement
The authors have nothing to disclose.
Figures




References
-
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127(5):1565–1577. - PubMed
-
- Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146(2):349–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

