The method quality of cross-over studies involved in Cochrane Systematic Reviews
- PMID: 25867772
- PMCID: PMC4395015
- DOI: 10.1371/journal.pone.0120519
The method quality of cross-over studies involved in Cochrane Systematic Reviews
Abstract
Background: It is possible that cross-over studies included in current systematic reviews are being inadequately assessed, because the current risk of bias tools do not consider possible biases specific to cross-over design. We performed this study to evaluate whether this was being done in cross-over studies included in Cochrane Systematic Reviews (CSRs).
Methods: We searched the Cochrane Library (up to 2013 issue 5) for CSRs that included at least one cross-over trial. Two authors independently undertook the study selection and data extraction. A random sample of the CSRs was selected and we evaluated whether the cross-over trials in these CSRs were assessed according to criteria suggested by the Cochrane handbook. In addition we reassessed the risk of bias of these cross-over trials by a checklist developed form the Cochrane handbook.
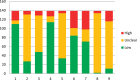
Results: We identified 688 CSRs that included one or more cross-over studies. We chose a random sample of 60 CSRs and these included 139 cross-over studies. None of these CSRs undertook a risk of bias assessment specific for cross-over studies. In fact items specific for cross-over studies were seldom considered anywhere in quality assessment of these CSRs. When we reassessed the risk of bias, including the 3 items specific to cross-over trials, of these 139 studies, a low risk of bias was judged for appropriate cross-over design in 110(79%), carry-over effects in 48(34%) and for reporting data in all stages of the trial in 114(82%).Assessment of biases in cross-over trials could affect the GRADE assessment of a review's findings.
Conclusion: The current Cochrane risk of bias tool is not adequate to assess cross-over studies. Items specific to cross-over trials leading to potential risk of bias are generally neglected in CSRs. A proposed check list for the evaluation of cross-over trials is provided.
Conflict of interest statement
Figures

References
-
- Senn SJ. Cross-over trials in clinical research Chichester: John Wiley; 2002; 1.
-
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991; 133(2): 144–153. - PubMed
-
- The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions, version 5.0.2. Available: www.cochrane-handbook.org. 2008; Accessed 29 May 2010.
-
- Cleophas TJ, De Vogel EM. Crossover studies are a better format for comparing equivalent treatments than parallel-group studies. Pharm World Sci. 1998; 20(3): 113–117. - PubMed
-
- Brown BW Jr. The crossover experiment for clinical trials. Biometrics. 1980; 36: 69–79. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

