An Electronic Tool for the Evaluation and Treatment of Sepsis in the ICU: A Randomized Controlled Trial
- PMID: 25867906
- PMCID: PMC4506222
- DOI: 10.1097/CCM.0000000000001020
An Electronic Tool for the Evaluation and Treatment of Sepsis in the ICU: A Randomized Controlled Trial
Abstract
Objectives: To determine whether addition of an electronic sepsis evaluation and management tool to electronic sepsis alerting improves compliance with treatment guidelines and clinical outcomes in septic ICU patients.
Design: A pragmatic randomized trial.
Setting: Medical and surgical ICUs of an academic, tertiary care medical center.
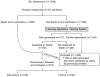
Patients: Four hundred and seven patients admitted during a 4-month period to the medical or surgical ICU with a diagnosis of sepsis established at the time of admission or in response to an electronic sepsis alert.
Interventions: Patients were randomized to usual care or the availability of an electronic tool capable of importing, synthesizing, and displaying sepsis-related data from the medical record, using logic rules to offer individualized evaluations of sepsis severity and response to therapy, informing users about evidence-based guidelines, and facilitating rapid order entry.
Measurements and main results: There was no difference between the electronic tool (218 patients) and usual care (189 patients) with regard to the primary outcome of time to completion of all indicated Surviving Sepsis Campaign 6-hour Sepsis Resuscitation Bundle elements (hazard ratio, 1.98; 95% CI, 0.75-5.20; p = 0.159) or time to completion of each element individually. ICU mortality, ICU-free days, and ventilator-free days did not differ between intervention and control. Providers used the tool to enter orders in only 28% of available cases.
Conclusions: A comprehensive electronic sepsis evaluation and management tool is feasible and safe but did not influence guideline compliance or clinical outcomes, perhaps due to low utilization.
Conflict of interest statement
Conflicts of Interest and Source of Funding: Study supported by grants 1RC1LM010310-01 from NIH, 1 UL1 RR024975 from NCRR/NIH, and CCF- 0424422 from NSF. All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. M.H.H. reported prior consulting for Biomereux and T.W.R. reported serving on an advisory board for Avisa Pharma, LLC and as a DSMB member for GlaxoSmithKline. Otherwise, the authors declare no potential conflicts of interest.
Figures


Comment in
-
Automated Sepsis Detection, Alert, and Clinical Decision Support: Act on It or Silence the Alarm?Crit Care Med. 2015 Aug;43(8):1776-7. doi: 10.1097/CCM.0000000000001099. Crit Care Med. 2015. PMID: 26181117 No abstract available.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. - PubMed
-
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA J Am Med Assoc. 1995;273(2):117–123. - PubMed
-
- Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28(2):108–121. doi: 10.1007/s00134-001-1143-z. - DOI - PubMed
-
- Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, Kline JA Emergency Medicine Shock Research Network investigators. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008;36(10):2734–2739. doi: 10.1097/CCM.0b013e318186f839. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

