Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications
- PMID: 25868399
- PMCID: PMC4427881
- DOI: 10.1038/mt.2015.44
Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications
Abstract
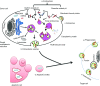
Mesenchymal stem (stromal) cells (MSCs) are multipotent cells with the ability to differentiate into several cell types, thus serving as a cell reservoir for regenerative medicine. Much of the current interest in therapeutic application of MSCs to various disease settings can be linked to their immunosuppressive and anti-inflammatory properties. One of the key mechanisms of MSC anti-inflammatory effects is the secretion of soluble factors with paracrine actions. Recently it has emerged that the paracrine functions of MSCs could, at least in part, be mediated by extracellular vesicles (EVs). EVs are predominantly released from the endosomal compartment and contain a cargo that includes miRNA, mRNA, and proteins from their cells of origin. Recent animal model-based studies suggest that EVs have significant potential as a novel alternative to whole cell therapies. Compared to their parent cells, EVs may have a superior safety profile and can be safely stored without losing function. In this article, we review current knowledge related to the potential use of MSC-derived EVs in various diseases and discuss the promising future for EVs as an alternative, cell-free therapy.
Figures

References
-
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. - PubMed
-
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. - PubMed
-
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. - PubMed
-
- Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, et al. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

