Foxp1-mediated programming of limb-innervating motor neurons from mouse and human embryonic stem cells
- PMID: 25868900
- PMCID: PMC4397664
- DOI: 10.1038/ncomms7778
Foxp1-mediated programming of limb-innervating motor neurons from mouse and human embryonic stem cells
Abstract
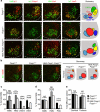
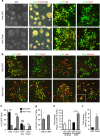
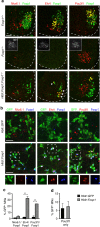
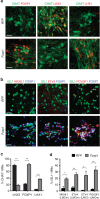
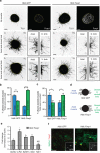
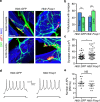
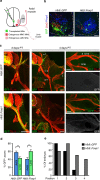
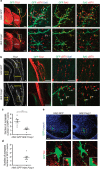
Spinal motor neurons (MNs) control diverse motor tasks including respiration, posture and locomotion that are disrupted by neurodegenerative diseases such as amyotrophic lateral sclerosis and spinal muscular atrophy. Methods directing MN differentiation from stem cells have been developed to enable disease modelling in vitro. However, most protocols produce only a limited subset of endogenous MN subtypes. Here we demonstrate that limb-innervating lateral motor column (LMC) MNs can be efficiently generated from mouse and human embryonic stem cells through manipulation of the transcription factor Foxp1. Foxp1-programmed MNs exhibit features of medial and lateral LMC MNs including expression of specific motor pool markers and axon guidance receptors. Importantly, they preferentially project axons towards limb muscle explants in vitro and distal limb muscles in vivo upon transplantation-hallmarks of bona fide LMC MNs. These results present an effective approach for generating specific MN populations from stem cells for studying MN development and disease.
Figures

References
-
- Frattini E. et al.. Pluripotent stem cell-based models of spinal muscular atrophy. Mol. Cell. Neurosci. 64C, 44–50 (2014) . - PubMed
-
- Sandoe J. & Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat. Neurosci. 16, 780–789 (2013) . - PubMed
-
- Winner B., Marchetto M. C., Winkler J. & Gage F. H. Human-induced pluripotent stem cells pave the road for a better understanding of motor neuron disease. Hum. Mol. Genet. 23, R27–R34 (2014) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

