The burrowing behavior of the nematode Caenorhabditis elegans: a new assay for the study of neuromuscular disorders
- PMID: 25868909
- PMCID: PMC4444045
- DOI: 10.1111/gbb.12217
The burrowing behavior of the nematode Caenorhabditis elegans: a new assay for the study of neuromuscular disorders
Abstract
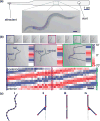
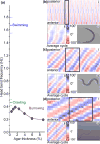
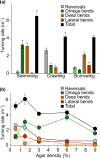
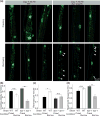
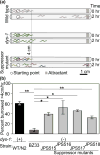
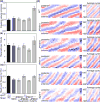
The nematode Caenorhabditis elegans has been a powerful model system for the study of key muscle genes relevant to human neuromuscular function and disorders. The behavioral robustness of C. elegans, however, has hindered its use in the study of certain neuromuscular disorders because many worm models of human disease show only subtle phenotypes while crawling. By contrast, in their natural habitat, C. elegans likely spends much of the time burrowing through the soil matrix. We developed a burrowing assay to challenge motor output by placing worms in agar-filled pipettes of increasing densities. We find that burrowing involves distinct kinematics and turning strategies from crawling that vary with the properties of the substrate. We show that mutants mimicking Duchenne muscular dystrophy by lacking a functional ortholog of the dystrophin protein, DYS-1, crawl normally but are severely impaired in burrowing. Muscular degeneration in the dys-1 mutant is hastened and exacerbated by burrowing, while wild type shows no such damage. To test whether neuromuscular integrity might be compensated genetically in the dys-1 mutant, we performed a genetic screen and isolated several suppressor mutants with proficient burrowing in a dys-1 mutant background. Further study of burrowing in C. elegans will enhance the study of diseases affecting neuromuscular integrity, and will provide insights into the natural behavior of this and other nematodes.
Keywords: Behavior; Caenorhabditis elegans; burrowing; dystrophin; nematode.
© 2015 John Wiley & Sons Ltd and International Behavioural and Neural Genetics Society.
Conflict of interest statement
Figures


References
-
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. - PubMed
-
- Croll NA. Components and patterns of in the behavior of the nematode Caenorhabditis elegans. J Zool. 1975;176:159–176.
-
- Culetto E, Sattelle DB. A role for Caenorhabditis elegans in understanding the function and interaction of human disease genes. Hum Mol Gen. 2000;9:869–877. - PubMed
-
- Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: Old and new players. Nat Rev Mol Cell Biol. 2006;7:762–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

