Tricyclic Antidepressant Amitriptyline-induced Glial Cell Line-derived Neurotrophic Factor Production Involves Pertussis Toxin-sensitive Gαi/o Activation in Astroglial Cells
- PMID: 25869129
- PMCID: PMC4447948
- DOI: 10.1074/jbc.M114.622415
Tricyclic Antidepressant Amitriptyline-induced Glial Cell Line-derived Neurotrophic Factor Production Involves Pertussis Toxin-sensitive Gαi/o Activation in Astroglial Cells
Abstract
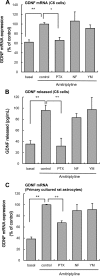
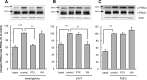
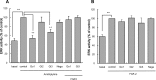
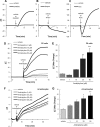
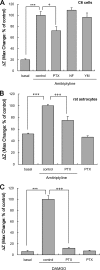
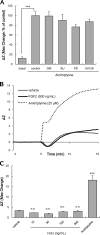
Further elaborating the mechanism of antidepressants, beyond modulation of monoaminergic neurotransmission, this study sought to elucidate the mechanism of amitriptyline-induced production of glial cell line-derived neurotrophic factor (GDNF) in astroglial cells. Previous studies demonstrated that an amitriptyline-evoked matrix metalloproteinase (MMP)/FGF receptor (FGFR)/FGFR substrate 2α (FRS2α)/ERK cascade is crucial for GDNF production, but how amitriptyline triggers this cascade remains unknown. MMP is activated by intracellular mediators such as G proteins, and this study sought to clarify the involvement of G protein signaling in amitriptyline-evoked GDNF production in rat C6 astroglial cells (C6 cells), primary cultured rat astrocytes, and normal human astrocytes. Amitriptyline-evoked GDNF mRNA expression and release were inhibited by pertussis toxin (PTX), a Gα(i/o) inhibitor, but not by NF449, a Gα(s) inhibitor, or YM-254890, a Gαq inhibitor. The activation of the GDNF production cascade (FGFR/FRS2α/ERK) was also inhibited by PTX. Deletion of Gα(ο1) and Gα(i3) by RNAi demonstrated that these G proteins play important roles in amitriptyline signaling. G protein activation was directly analyzed by electrical impedance-based biosensors (CellKey(TM) assay), using a label-free (without use of fluorescent proteins/probes or radioisotopes) and real time approach. Amitriptyline increased impedance, indicating Gα(i/o) activation that was suppressed by PTX treatment. The impedance evoked by amitriptyline was not affected by inhibitors of the GDNF production cascade. Furthermore, FGF2 treatment did not elicit any effect on impedance, indicating that amitriptyline targets PTX-sensitive Gα(i/o) upstream of the MMP/FGFR/FRS2α/ERK cascade. These results suggest novel targeting for the development of antidepressants.
Keywords: Cellkey assay; G protein; antidepressant; astrocyte; biosensor; depression; glial cell.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Belmaker R. H., Agam G. (2008) Major depressive disorder. N. Engl. J. Med. 358, 55–68 - PubMed
-
- Allen N. J., Barres B. A. (2009) Neuroscience: Glia–more than just brain glue. Nature 457, 675–677 - PubMed
-
- Bespalov M. M., Saarma M. (2007) GDNF family receptor complexes are emerging drug targets. Trends Pharmacol. Sci. 28, 68–74 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

