Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex
- PMID: 25869275
- PMCID: PMC4605847
- DOI: 10.1007/s00429-015-1040-9
Transgene expression in the Nop-tTA driver line is not inherently restricted to the entorhinal cortex
Abstract
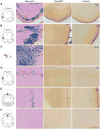
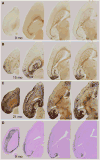
The entorhinal cortex (EC) plays a central role in episodic memory and is among the earliest sites of neurodegeneration and neurofibrillary tangle formation in Alzheimer's disease. Given its importance in memory and dementia, the ability to selectively modulate gene expression or neuronal function in the EC is of widespread interest. To this end, several recent studies have taken advantage of a transgenic line in which the tetracycline transactivator (tTA) was placed under control of the neuropsin (Nop) promoter to limit transgene expression within the medial EC and pre-/parasubiculum. Although the utility of this driver is contingent on its spatial specificity, no detailed neuroanatomical analysis of its expression has yet been conducted. We therefore undertook a systematic analysis of Nop-tTA expression using a lacZ reporter and have made the complete set of histological sections available through the Rodent Brain Workbench tTA atlas, www.rbwb.org . Our findings confirm that the highest density of tTA expression is found in the EC and pre-/parasubiculum, but also reveal considerable expression in several other cortical areas. Promiscuous transgene expression may account for the appearance of pathological protein aggregates outside of the EC in mouse models of Alzheimer's disease using this driver, as we find considerable overlap between sites of delayed amyloid deposition and regions with sparse β-galactosidase reporter labeling. While different tet-responsive lines can display individual expression characteristics, our results suggest caution when designing experiments that depend on precise localization of gene products controlled by the Nop-tTA or other spatially restrictive transgenic drivers.
Keywords: Ectopic expression; Rodent brain atlas; Tet-lacZ reporter; Tet-off transgenic; Tetracycline transactivator.
Conflict of interest statement
Figures





Similar articles
-
Strain background influences neurotoxicity and behavioral abnormalities in mice expressing the tetracycline transactivator.J Neurosci. 2012 Aug 1;32(31):10574-86. doi: 10.1523/JNEUROSCI.0893-12.2012. J Neurosci. 2012. PMID: 22855807 Free PMC article.
-
Atlas of transgenic Tet-Off Ca2+/calmodulin-dependent protein kinase II and prion protein promoter activity in the mouse brain.Neuroimage. 2011 Feb 14;54(4):2603-11. doi: 10.1016/j.neuroimage.2010.11.032. Epub 2010 Nov 18. Neuroimage. 2011. PMID: 21093594
-
Tetracycline inducible gene manipulation in serotonergic neurons.PLoS One. 2012;7(5):e38193. doi: 10.1371/journal.pone.0038193. Epub 2012 May 31. PLoS One. 2012. PMID: 22693598 Free PMC article.
-
Brain-wide distribution of reporter expression in five transgenic tetracycline-transactivator mouse lines.Sci Data. 2019 Feb 26;6:190028. doi: 10.1038/sdata.2019.28. Sci Data. 2019. PMID: 30806643 Free PMC article.
-
Using conditional mutagenesis to study the brain.Biol Psychiatry. 2003 Dec 1;54(11):1125-33. doi: 10.1016/s0006-3223(03)00467-0. Biol Psychiatry. 2003. PMID: 14643079 Review.
Cited by
-
Impaired Recall of Positional Memory following Chemogenetic Disruption of Place Field Stability.Cell Rep. 2016 Jul 19;16(3):793-804. doi: 10.1016/j.celrep.2016.06.032. Epub 2016 Jun 30. Cell Rep. 2016. PMID: 27373150 Free PMC article.
-
Activity disruption causes degeneration of entorhinal neurons in a mouse model of Alzheimer's circuit dysfunction.Elife. 2022 Dec 5;11:e83813. doi: 10.7554/eLife.83813. Elife. 2022. PMID: 36468693 Free PMC article.
-
Does proteopathic tau propagate trans-synaptically in the brain?Mol Neurodegener. 2022 Mar 16;17(1):21. doi: 10.1186/s13024-022-00527-x. Mol Neurodegener. 2022. PMID: 35296344 Free PMC article. No abstract available.
-
Trans-Synaptic Spread of Amyloid-β in Alzheimer's Disease: Paths to β-Amyloidosis.Neural Plast. 2017;2017:5281829. doi: 10.1155/2017/5281829. Epub 2017 Dec 24. Neural Plast. 2017. PMID: 29435372 Free PMC article. Review.
-
Patterns of Invasive Growth in Malignant Gliomas-The Hippocampus Emerges as an Invasion-Spared Brain Region.Neoplasia. 2018 Jul;20(7):643-656. doi: 10.1016/j.neo.2018.04.001. Epub 2018 May 21. Neoplasia. 2018. PMID: 29793116 Free PMC article.
References
-
- Bjaalie JG, Leergaard TB. Three-dimensional computerized reconstruction from serial sections: cell populations, regions, and whole brain. In: Zaborszky L, Wouterlood FG, Lanciego JL, editors. Neuroanatomical tract tracing: molecules, neurons and systems. 3rd. Springer/Kluwer/Plenum; 2006. pp. 530–565.
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

