α-Globin as a molecular target in the treatment of β-thalassemia
- PMID: 25869286
- PMCID: PMC4497969
- DOI: 10.1182/blood-2015-03-633594
α-Globin as a molecular target in the treatment of β-thalassemia
Abstract
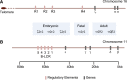
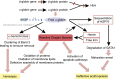
The thalassemias, together with sickle cell anemia and its variants, are the world's most common form of inherited anemia, and in economically undeveloped countries, they still account for tens of thousands of premature deaths every year. In developed countries, treatment of thalassemia is also still far from ideal, requiring lifelong transfusion or allogeneic bone marrow transplantation. Clinical and molecular genetic studies over the course of the last 50 years have demonstrated how coinheritance of modifier genes, which alter the balance of α-like and β-like globin gene expression, may transform severe, transfusion-dependent thalassemia into relatively mild forms of anemia. Most attention has been paid to pathways that increase γ-globin expression, and hence the production of fetal hemoglobin. Here we review the evidence that reduction of α-globin expression may provide an equally plausible approach to ameliorating clinically severe forms of β-thalassemia, and in particular, the very common subgroup of patients with hemoglobin E β-thalassemia that makes up approximately half of all patients born each year with severe β-thalassemia.
© 2015 by The American Society of Hematology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

