Ginkgolide B Protects Neurons from Ischemic Injury by Inhibiting the Expression of RTP801
- PMID: 25869596
- PMCID: PMC11486257
- DOI: 10.1007/s10571-015-0189-3
Ginkgolide B Protects Neurons from Ischemic Injury by Inhibiting the Expression of RTP801
Abstract
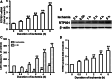
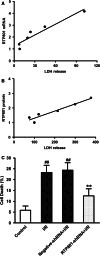
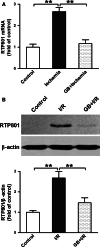
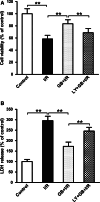
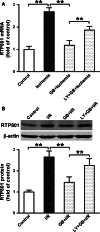
RTP801 (also known as REDD1), a stress-related protein, is induced by several environmental stresses such as ischemia and cigarette smoke. Although ischemia can dramatically up-regulate RTP801 expression in brain ischemia, up to now, the exact relation between RTP801 and neuronal death in ischemia is poorly understood. In the current study, using oxygen and glucose deprivation as an in vitro ischemic model in primary cultured cortical neurons, we found that the expression of RTP801 increased progressively with prolongation of ischemic duration, in which the expression of RTP801 is positively correlated with the release of lactate dehydrogenase (LDH) in neurons, and knockdown of RTP801 promoted neuronal survival in ischemia-reperfusion. It was further found that ginkgolide B (GB) could significantly increase cell viability and decrease LDH release, and at the same time reduce the levels of RTP801 mRNA and protein in neurons after ischemia and reperfusion. Moreover, GB-induced reduction in expression of RTP801 was blocked by application of LY294002, a specific inhibitor of phosphatidylinositol 3-kinase (PI3K). These results demonstrate that RTP801 could play a detrimental role on neurons in ischemia, and GB might protect neurons against ischemic injury by inhibiting RTP801 expression via PI3K pathway.
Keywords: Ginkgolide B; Ischemia; Ischemia–Reperfusion; Neuron; Phosphatidylinositol 3-kinase; RTP801.
Conflict of interest statement
The authors state no conflict of interest.
Figures
References
-
- Bate C, Kempster S, Williams A (2006) Platelet-activating factor antagonists protect amyloid-beta damaged neurons from microglia-mediated death. Neuropharmacology 51(2):173–181 - PubMed
-
- Chung HS, Harris A, Kristinsson JK, Ciulla TA, Kagemann C, Ritch R (1999) Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther 15(3):233–240 - PubMed
-
- Fang D, Li Z, Zhong-ming Q, Mei WX, Ho YW, Yuan XW, Ya K (2008) Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim Biophys Acta 1782(11):658–663 - PubMed
-
- Fang W, Deng Y, Li Y, Shang E, Fang F, Lv P, Bai L, Qi Y, Yan F, Mao L (2010) Blood brain barrier permeability and therapeutic time window of ginkgolide B in ischemia–reperfusion injury. Eur J Pharm Sci 39(1–3):8–14 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

