Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance
- PMID: 25869855
- PMCID: PMC4450121
- DOI: 10.1016/j.ydbio.2015.03.019
Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance
Abstract
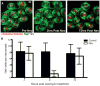
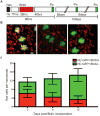
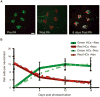
We have examined lateral line hair cell and support cell maintenance in adult zebrafish when growth is largely complete. We demonstrate that adult zebrafish not only replenish hair cells after a single instance of hair cell damage, but also maintain hair cells and support cells after multiple rounds of damage and regeneration. We find that hair cells undergo continuous turnover in adult zebrafish in the absence of damage. We identify mitotically-distinct support cell populations and show that hair cells regenerate from underlying support cells in a region-specific manner. Our results demonstrate that there are two distinct support cell populations in the lateral line, which may help explain why zebrafish hair cell regeneration is extremely robust, retained throughout life, and potentially unlimited in regenerative capacity.
Keywords: Adult zebrafish; Hair cells; Lateral line; Neuromasts; Regeneration.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205(1):17–20. - PubMed
-
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann NY Acad Sci. 1996;781:59–70. - PubMed
-
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7(6):656–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

