Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide
- PMID: 25870032
- PMCID: PMC4412688
- DOI: 10.1007/s00228-015-1832-0
Narrow therapeutic index drugs: a clinical pharmacological consideration to flecainide
Abstract
Purpose: The therapeutic index (TI) is the range of doses at which a medication is effective without unacceptable adverse events. Drugs with a narrow TI (NTIDs) have a narrow window between their effective doses and those at which they produce adverse toxic effects. Generic drugs may be substituted for brand-name drugs provided that they meet the recommended bioequivalence (BE) limits. However, an appropriate range of BE for NTIDs is essential to define due to the potential for ineffectiveness or adverse events. Flecainide is an antiarrhythmic agent that has the potential to be considered an NTID. This review aims to evaluate the literature surrounding guidelines on generic substitution for NTIDs and to evaluate the evidence for flecainide to be considered an NTID.
Methods: A review of recommendations from various regulatory authorities regarding BE and NTIDs, and publications regarding the NTID characteristics of flecainide, was carried out.
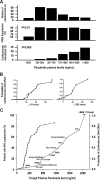
Results: Regulatory authorities generally recommend reduced BE limits for NTIDs. Some, but not all, regulatory authorities specify flecainide as an NTID. The literature review demonstrated that flecainide displays NTID characteristics including a steep drug dose-response relationship for safety and efficacy, a need for therapeutic drug monitoring of pharmacokinetic (PK) or pharmacodynamics measures and intra-subject variability in its PK properties.
Conclusions: There is much evidence for flecainide to be considered an NTID based on both preclinical and clinical data. A clear understanding of the potential of proarrhythmic effects or lack of efficacy, careful patient selection and regular monitoring are essential for the safe and rational administration of flecainide.
Figures

References
-
- Katzung B, Masters S, Trevor A. Basic and clinical pharmacology. 12. New York: Companies, Inc.; 2012.
-
- Reiffel JA. Formulation substitution and other pharmacokinetic variability: underappreciated variables affecting antiarrhythmic efficacy and safety in clinical practice. Am J Cardiol. 2000;85:46D–52D. - PubMed
-
- Reiffel JA. Issues in the use of generic antiarrhythmic drugs. Curr Opin Cardiol. 2001;16:23–29. - PubMed
-
- Buehler G (2010) History of bioequivalence for critical dose drugs
-
- CFR—Code of Federal Register 21, section 320.33 (21CFR320.33) (2014) Criteria and evidence to assess actual or potential bioequivalence problems
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

