Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical Models
- PMID: 25870145
- PMCID: PMC4605607
- DOI: 10.1158/0008-5472.CAN-14-3167
Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical Models
Abstract
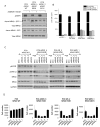
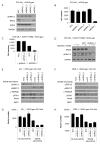
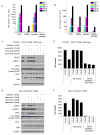
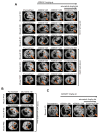
Resistance to targeted EGFR inhibitors is likely to develop in EGFR-mutant lung cancers. Early identification of innate or acquired resistance mechanisms to these agents is essential to direct development of future therapies. We describe the detection of heterogeneous mechanisms of resistance within populations of EGFR-mutant cells (PC9 and/or NCI-H1975) with acquired resistance to current and newly developed EGFR tyrosine kinase inhibitors, including AZD9291. We report the detection of NRAS mutations, including a novel E63K mutation, and a gain of copy number of WT NRAS or WT KRAS in cell populations resistant to gefitinib, afatinib, WZ4002, or AZD9291. Compared with parental cells, a number of resistant cell populations were more sensitive to inhibition by the MEK inhibitor selumetinib (AZD6244; ARRY-142886) when treated in combination with the originating EGFR inhibitor. In vitro, a combination of AZD9291 with selumetinib prevented emergence of resistance in PC9 cells and delayed resistance in NCI-H1975 cells. In vivo, concomitant dosing of AZD9291 with selumetinib caused regression of AZD9291-resistant tumors in an EGFRm/T790M transgenic model. Our data support the use of a combination of AZD9291 with a MEK inhibitor to delay or prevent resistance to AZD9291 in EGFRm and/or EGFRm/T790M tumors. Furthermore, these findings suggest that NRAS modifications in tumor samples from patients who have progressed on current or EGFR inhibitors in development may support subsequent treatment with a combination of EGFR and MEK inhibition.
©2015 American Association for Cancer Research.
Conflict of interest statement
A patent relating to EGFR T790M mutation testing was licensed on behalf of William Pao and others by Memorial Sloan-Kettering Cancer Center to MolecularMD. William Pao received research funding from AstraZeneca. C.A. Eberlein, D Stetson, A.A Markovets, Z Lai, K.J Al-Kadhimi, S.J Ross, M.J Ahdesmaki, A Ahmed, C.H Barnes, H Brown, P.D Smith, J.R Dry, G Beran, K.S Thress, B Dougherty, D.A.E. Cross are employees of AstraZeneca.
Figures

References
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57. - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

