Mechanically-driven phase separation in a growing bacterial colony
- PMID: 25870260
- PMCID: PMC4418877
- DOI: 10.1073/pnas.1504948112
Mechanically-driven phase separation in a growing bacterial colony
Abstract
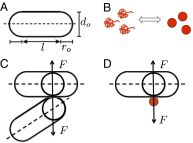
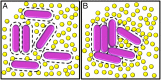
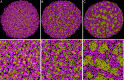
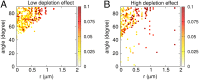
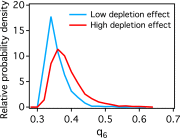
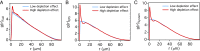
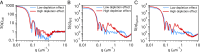
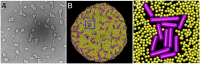
Secretion of extracellular polymeric substances (EPSs) by growing bacteria is an integral part of forming biofilm-like structures. In such dense systems, mechanical interactions among the structural components can be expected to significantly contribute to morphological properties. Here, we use a particle-based modeling approach to study the self-organization of nonmotile rod-shaped bacterial cells growing on a solid substrate in the presence of self-produced EPSs. In our simulation, all of the components interact mechanically via repulsive forces, occurring as the bacterial cells grow and divide (via consuming diffusing nutrient) and produce EPSs. Based on our simulation, we show that mechanical interactions control the collective behavior of the system. In particular, we find that the presence of nonadsorbing EPSs can lead to spontaneous aggregation of bacterial cells by a depletion attraction and thereby generates phase separated patterns in the nonequilibrium growing colony. Both repulsive interactions between cell and EPSs and the overall concentration of EPSs are important factors in the self-organization in a nonequilibrium growing colony. Furthermore, we investigate the interplay of mechanics with the nutrient diffusion and consumption by bacterial cells and observe that suppression of branch formation occurs due to EPSs compared with the case where no EPS is produced.
Keywords: biofilms; depletion interaction; extracellular polymeric substance; mechanical interaction; phase separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mechanically driven growth of quasi-two-dimensional microbial colonies.Phys Rev Lett. 2013 Oct 18;111(16):168101. doi: 10.1103/PhysRevLett.111.168101. Epub 2013 Oct 14. Phys Rev Lett. 2013. PMID: 24182305
-
Interplay of cell motility and self-secreted extracellular polymeric substance induced depletion effects on spatial patterning in a growing microbial colony.Soft Matter. 2023 Nov 1;19(42):8136-8149. doi: 10.1039/d3sm01144e. Soft Matter. 2023. PMID: 37847026
-
Simulation of various biofilm fractal morphologies by agent-based model.Colloids Surf B Biointerfaces. 2023 Jul;227:113352. doi: 10.1016/j.colsurfb.2023.113352. Epub 2023 May 13. Colloids Surf B Biointerfaces. 2023. PMID: 37196464
-
The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development.Environ Microbiol Rep. 2013 Dec;5(6):778-86. doi: 10.1111/1758-2229.12085. Epub 2013 Jul 25. Environ Microbiol Rep. 2013. PMID: 24249286 Review.
-
Modeling of biofilm systems: a review.Adv Biochem Eng Biotechnol. 2014;146:53-76. doi: 10.1007/10_2014_275. Adv Biochem Eng Biotechnol. 2014. PMID: 25163572 Review.
Cited by
-
Viscoelasticity variation in a biofilm-mediated Bacillus subtilis suspension induced by adding polyethylene glycol.Eur Biophys J. 2019 Oct;48(7):599-608. doi: 10.1007/s00249-019-01385-0. Epub 2019 Jul 6. Eur Biophys J. 2019. PMID: 31280338
-
Collective protection against the type VI secretion system in bacteria.ISME J. 2023 Jul;17(7):1052-1062. doi: 10.1038/s41396-023-01401-4. Epub 2023 Apr 24. ISME J. 2023. PMID: 37095301 Free PMC article.
-
Mechanics of biofilms formed of bacteria with fimbriae appendages.PLoS One. 2020 Dec 8;15(12):e0243280. doi: 10.1371/journal.pone.0243280. eCollection 2020. PLoS One. 2020. PMID: 33290393 Free PMC article.
-
Bacterial growth: a statistical physicist's guide.Rep Prog Phys. 2019 Jan;82(1):016601. doi: 10.1088/1361-6633/aae546. Epub 2018 Oct 1. Rep Prog Phys. 2019. PMID: 30270850 Free PMC article. Review.
-
Molecular choreography of sludge extracellular polymeric substances-From biomolecule identification to energetics and assembly dynamics.PNAS Nexus. 2025 May 17;4(5):pgaf157. doi: 10.1093/pnasnexus/pgaf157. eCollection 2025 May. PNAS Nexus. 2025. PMID: 40438222 Free PMC article.
References
-
- Cross MC, Hohenberg PC. Pattern formation outside of equilibrium. Rev Mod Phys. 1993;65(3):851–1123.
-
- Vicsek T, Zafeiris A. Collective motion. Phys Rep. 2012;517(3-4):71–140.
-
- Marchetti MC, et al. Hydrodynamics of soft active matter. Rev Mod Phys. 2013;85(3):1143–1189.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

