Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns
- PMID: 25870275
- PMCID: PMC4418913
- DOI: 10.1073/pnas.1504154112
Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns
Abstract
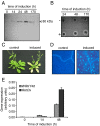
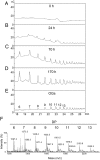
Oligogalacturonides (OGs) are fragments of pectin that activate plant innate immunity by functioning as damage-associated molecular patterns (DAMPs). We set out to test the hypothesis that OGs are generated in planta by partial inhibition of pathogen-encoded polygalacturonases (PGs). A gene encoding a fungal PG was fused with a gene encoding a plant polygalacturonase-inhibiting protein (PGIP) and expressed in transgenic Arabidopsis plants. We show that expression of the PGIP-PG chimera results in the in vivo production of OGs that can be detected by mass spectrometric analysis. Transgenic plants expressing the chimera under control of a pathogen-inducible promoter are more resistant to the phytopathogens Botrytis cinerea, Pectobacterium carotovorum, and Pseudomonas syringae. These data provide strong evidence for the hypothesis that OGs released in vivo act as a DAMP signal to trigger plant immunity and suggest that controlled release of these molecules upon infection may be a valuable tool to protect plants against infectious diseases. On the other hand, elevated levels of expression of the chimera cause the accumulation of salicylic acid, reduced growth, and eventually lead to plant death, consistent with the current notion that trade-off occurs between growth and defense.
Keywords: DAMPs; PGIP; oligogalacturonides; plant immunity; polygalacturonase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

