Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure
- PMID: 25870291
- PMCID: PMC4426460
- DOI: 10.1073/pnas.1504778112
Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure
Abstract
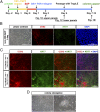
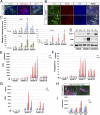
Human pluripotent stem cells (PSCs) show epiblast-type pluripotency that is maintained with ACTIVIN/FGF2 signaling. Here, we report the acquisition of a unique stem cell phenotype by both human ES cells (hESCs) and induced pluripotent stem cells (iPSCs) in response to transient (24-36 h) exposure to bone morphogenetic protein 4 (BMP4) plus inhibitors of ACTIVIN signaling (A83-01) and FGF2 (PD173074), followed by trypsin dissociation and recovery of colonies capable of growing on a gelatin substratum in standard medium for human PSCs at low but not high FGF2 concentrations. The self-renewing cell lines stain weakly for CDX2 and strongly for NANOG, can be propagated clonally on either Matrigel or gelatin, and are morphologically distinct from human PSC progenitors on either substratum but still meet standard in vitro criteria for pluripotency. They form well-differentiated teratomas in immune-compromised mice that secrete human chorionic gonadotropin (hCG) into the host mouse and include small areas of trophoblast-like cells. The cells have a distinct transcriptome profile from the human PSCs from which they were derived (including higher expression of NANOG, LEFTY1, and LEFTY2). In nonconditioned medium lacking FGF2, the colonies spontaneously differentiated along multiple lineages, including trophoblast. They responded to PD173074 in the absence of both FGF2 and BMP4 by conversion to trophoblast, and especially syncytiotrophoblast, whereas an A83-01/PD173074 combination favored increased expression of HLA-G, a marker of extravillous trophoblast. Together, these data suggest that the cell lines exhibit totipotent potential and that BMP4 can prime human PSCs to a self-renewing alternative state permissive for trophoblast development. The results may have implications for regulation of lineage decisions in the early embryo.
Keywords: biological sciences; developmental biology; pluripotent stem cells; totipotent; trophoblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Comparison of Four Protocols for In Vitro Differentiation of Human Embryonic Stem Cells into Trophoblast Lineages by BMP4 and Dual Inhibition of Activin/Nodal and FGF2 Signaling.Reprod Sci. 2024 Jan;31(1):173-189. doi: 10.1007/s43032-023-01334-5. Epub 2023 Sep 1. Reprod Sci. 2024. PMID: 37658178 Free PMC article.
-
Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4.Proc Natl Acad Sci U S A. 2013 Mar 26;110(13):E1212-21. doi: 10.1073/pnas.1303094110. Epub 2013 Mar 14. Proc Natl Acad Sci U S A. 2013. PMID: 23493551 Free PMC article.
-
BMP10 as a potent inducer of trophoblast differentiation in human embryonic and induced pluripotent stem cells.Biomaterials. 2013 Dec;34(38):9789-802. doi: 10.1016/j.biomaterials.2013.08.084. Epub 2013 Sep 23. Biomaterials. 2013. PMID: 24070570
-
The role of BMP4 signaling in trophoblast emergence from pluripotency.Cell Mol Life Sci. 2022 Jul 25;79(8):447. doi: 10.1007/s00018-022-04478-w. Cell Mol Life Sci. 2022. PMID: 35877048 Free PMC article. Review.
-
Model systems for studying trophoblast differentiation from human pluripotent stem cells.Cell Tissue Res. 2012 Sep;349(3):809-24. doi: 10.1007/s00441-012-1371-2. Epub 2012 Mar 17. Cell Tissue Res. 2012. PMID: 22427062 Free PMC article. Review.
Cited by
-
iPSC-based modeling of preeclampsia identifies epigenetic defects in extravillous trophoblast differentiation.iScience. 2024 Mar 25;27(4):109569. doi: 10.1016/j.isci.2024.109569. eCollection 2024 Apr 19. iScience. 2024. PMID: 38623329 Free PMC article.
-
Lineage-Specific Mesenchymal Stromal Cells Derived from Human iPSCs Showed Distinct Patterns in Transcriptomic Profile and Extracellular Vesicle Production.Adv Sci (Weinh). 2024 Jul;11(28):e2308975. doi: 10.1002/advs.202308975. Epub 2024 May 17. Adv Sci (Weinh). 2024. PMID: 38757640 Free PMC article.
-
A stepwise model of reaction-diffusion and positional information governs self-organized human peri-gastrulation-like patterning.Development. 2017 Dec 1;144(23):4298-4312. doi: 10.1242/dev.149658. Epub 2017 Sep 4. Development. 2017. PMID: 28870989 Free PMC article.
-
Exploring maternal-fetal interface with in vitro placental and trophoblastic models.Front Cell Dev Biol. 2023 Nov 14;11:1279227. doi: 10.3389/fcell.2023.1279227. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38033854 Free PMC article. Review.
-
Deconstructing the pluripotency gene regulatory network.Nat Cell Biol. 2018 Apr;20(4):382-392. doi: 10.1038/s41556-018-0067-6. Epub 2018 Mar 28. Nat Cell Biol. 2018. PMID: 29593328 Free PMC article. Review.
References
-
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4(6):487–492. - PubMed
-
- Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–195. - PubMed
-
- Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–199. - PubMed
-
- Greber B, et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6(3):215–226. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

