Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro
- PMID: 25870293
- PMCID: PMC4443325
- DOI: 10.1073/pnas.1504393112
Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro
Erratum in
-
Correction for Bardy et al., Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):E3312. doi: 10.1073/pnas.1509741112. Epub 2015 May 28. Proc Natl Acad Sci U S A. 2015. PMID: 26023185 Free PMC article. No abstract available.
-
Correction to Supporting Information for Bardy et al., Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2512898122. doi: 10.1073/pnas.2512898122. Epub 2025 Jun 13. Proc Natl Acad Sci U S A. 2025. PMID: 40512729 Free PMC article. No abstract available.
Abstract
Human cell reprogramming technologies offer access to live human neurons from patients and provide a new alternative for modeling neurological disorders in vitro. Neural electrical activity is the essence of nervous system function in vivo. Therefore, we examined neuronal activity in media widely used to culture neurons. We found that classic basal media, as well as serum, impair action potential generation and synaptic communication. To overcome this problem, we designed a new neuronal medium (BrainPhys basal + serum-free supplements) in which we adjusted the concentrations of inorganic salts, neuroactive amino acids, and energetic substrates. We then tested that this medium adequately supports neuronal activity and survival of human neurons in culture. Long-term exposure to this physiological medium also improved the proportion of neurons that were synaptically active. The medium was designed to culture human neurons but also proved adequate for rodent neurons. The improvement in BrainPhys basal medium to support neurophysiological activity is an important step toward reducing the gap between brain physiological conditions in vivo and neuronal models in vitro.
Keywords: BrainPhys; induced pluripotent stem cells; neurobasal DMEM; neuromedium; tissue culture milieu.
Conflict of interest statement
Conflict of interest statement: The Salk Institute, C.B., and F.H.G. have filed a patent for the new BrainPhys medium described in this paper.
Figures
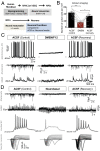
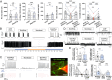
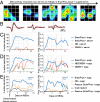
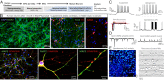


Comment in
-
Reconstructing the neuronal milieu intérieur.Proc Natl Acad Sci U S A. 2015 May 19;112(20):6250-1. doi: 10.1073/pnas.1506303112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964319 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

