Conditional targeting of medium spiny neurons in the striatal matrix
- PMID: 25870547
- PMCID: PMC4375991
- DOI: 10.3389/fnbeh.2015.00071
Conditional targeting of medium spiny neurons in the striatal matrix
Abstract
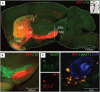
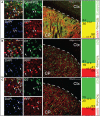
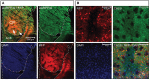
The striatum serves as the main input to the basal ganglia, and is key for the regulation of motor behaviors, compulsion, addiction, and various cognitive and emotional states. Its deterioration is associated with degenerative disorders such as Huntington's disease. Despite its apparent anatomical uniformity, it consists of intermingled cell populations, which have precluded straightforward anatomical sub-classifications adhering to functional dissections. Approximately 95% of the striatal neurons are inhibitory projection neurons termed medium spiny neurons (MSNs). They are commonly classified according to their expression of either dopamine receptor D1 or D2, which also determines their axonal projection patterns constituting the direct and indirect pathway in the basal ganglia. Immunohistochemical patterns have further indicated compartmentalization of the striatum to the striosomes and the surrounding matrix, which integrate MSNs of both the D1 and D2 type. Here, we present a transgenic mouse line, Gpr101-Cre, with Cre recombinase activity localized to matrix D1 and D2 MSNs. Using two Gpr101-Cre founder lines with different degrees of expression in the striatum, we conditionally deleted the vesicular inhibitory amino acid transporter (VIAAT), responsible for storage of GABA and glycine in synaptic vesicles. Partial ablation of VIAAT (in ~36% of MSNs) resulted in elevated locomotor activity compared to control mice, when provoked with the monoamine reuptake inhibitor cocaine. Near complete targeting of matrix MSNs led to profoundly changed motor behaviors, which increased in severity as the mice aged. Moreover, these mice had exaggerated muscle rigidity, retarded growth, increased rate of spontaneous deaths, and defective memory. Therefore, our data provide a link between dysfunctional GABA signaling of matrix MSNs to specific behavioral alterations, which are similar to the symptoms of Huntington's disease.
Keywords: GABA; SLC32A1; behavior; huntington's disease; matrix; medium spiny neuron; patches; striosome.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

