Antifungal compounds from cyanobacteria
- PMID: 25871291
- PMCID: PMC4413203
- DOI: 10.3390/md13042124
Antifungal compounds from cyanobacteria
Abstract
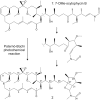
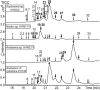
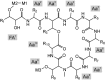
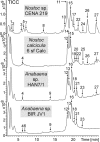
Cyanobacteria are photosynthetic prokaryotes found in a range of environments. They are infamous for the production of toxins, as well as bioactive compounds, which exhibit anticancer, antimicrobial and protease inhibition activities. Cyanobacteria produce a broad range of antifungals belonging to structural classes, such as peptides, polyketides and alkaloids. Here, we tested cyanobacteria from a wide variety of environments for antifungal activity. The potent antifungal macrolide scytophycin was detected in Anabaena sp. HAN21/1, Anabaena cf. cylindrica PH133, Nostoc sp. HAN11/1 and Scytonema sp. HAN3/2. To our knowledge, this is the first description of Anabaena strains that produce scytophycins. We detected antifungal glycolipopeptide hassallidin production in Anabaena spp. BIR JV1 and HAN7/1 and in Nostoc spp. 6sf Calc and CENA 219. These strains were isolated from brackish and freshwater samples collected in Brazil, the Czech Republic and Finland. In addition, three cyanobacterial strains, Fischerella sp. CENA 298, Scytonema hofmanni PCC 7110 and Nostoc sp. N107.3, produced unidentified antifungal compounds that warrant further characterization. Interestingly, all of the strains shown to produce antifungal compounds in this study belong to Nostocales or Stigonematales cyanobacterial orders.
Figures
References
-
- Whitton B.A., Potts M. Introduction to cyanobacteria. In: Whitton B.A., Potts M., editors. The Ecology of Cyanobacteria. Their Diversity in Time and Space. Kluwer Academic; Dodrecht, The Netherlands: 2000. pp. 61–120.
-
- Kajiyama S., Kanzaki H., Kawazu K., Kobayashi A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998;39:3737–3740. doi: 10.1016/S0040-4039(98)00573-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

