A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives
- PMID: 25871371
- PMCID: PMC6272598
- DOI: 10.3390/molecules20046520
A facile synthesis and antimicrobial activity evaluation of sydnonyl-substituted thiazolidine derivatives
Abstract
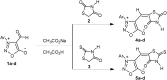
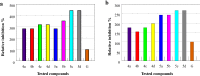
Some new sydnonyl-substituted thiazolidine derivatives were synthesized in high yields by the modified Knoevenagel condensation of 3-aryl-4-formylsydnones with thiazolidine-2,4-dione and 2-thioxo-thiazolidine-4-one, respectively. All the synthesized thiazolidine derivatives were screened by paper-disc method to identify their antimicrobial activities against three bacteria viz. Staphylococcus aureus, Proteus vulgaris and Escherichia coli, and two fungal cultures viz. Aspergillus niger and Penicillium citrinum. The reference drugs were Norfloxacin and Griseofulvin, respectively. The screening data indicated that the tested sydnonyl-substituted thiazolidine derivatives exhibited no obvious antibacterial activity compared with the standard drug Norfloxacin. However, thiazolidine derivatives displayed significant antifungal activities against Penicillium citrinum and Aspergillus niger. Notably, all of the tested compounds showed growth inhibitory activity 1.5-4.4 times higher than that of the standard drug Griseofulvin against the two fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ban J.O., Kwak D.H., Oh J.H., Park E.J., Cho M.C., Song H.S., Song M.J., Han S.B., Moon D.C., Kang K.W., et al. Suppression of NF-κB and GSK-3β is involved in colon cancer cell growth inhibition by the PPAR agonist troglitazone. Chem. Biol. Interact. 2010;188:75–85. - PubMed
-
- El-Gaby M.S.A., Ismail Z.H., Abdel-Gawad S.M., Aly H.M., Ghorab M.M. Synthesis of thiazolidine and thiophene derivatives for evaluation as anticancer agents. Phosphorus Sulfur Silicon Relat. Elem. 2009;184:2645–2654. doi: 10.1080/10426500802561096. - DOI
-
- Beharry Z., Zemskova M., Mahajan S., Zhang F., Ma J., Xia Z., Lilly M., Smith C.D., Kraft A.S. Novel benzylidene-thiazolidine-2,4-diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells. Mol. Cancer Ther. 2009;8:1473–1483. doi: 10.1158/1535-7163.MCT-08-1037. - DOI - PMC - PubMed
-
- Barros F.W.A., Silva T.G., da Rocha Pitta M.G., Bezerra D.P., Costa-Lotufo L.V., de Moraes M.O., Pessoa C., de Moura M.A.F.B., de Abreu F.C., de Lima M.C.A., et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorg. Med. Chem. 2012;20:3533–3539. doi: 10.1016/j.bmc.2012.04.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

